UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13A-16 OR 15D-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the month of September 2024
Commission File Number: 001-40010
Pharvaris N.V.
(Translation of registrant’s name into English)
Emmy Noetherweg 2
2333 BK Leiden
The Netherlands
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
PHARVARIS N.V.
Pharvaris Provides Business Update and Expands Development Program for Deucrictibant
On September 5, 2024, Pharvaris N.V. (the “Company”) issued a press release titled “Pharvaris Provides Business Update and Expands Development Program for Deucrictibant” which announced the planned initiation of CHAPTER-3, the pivotal Phase 3 study of deucrictibant for the prophylactic treatment of HAE by the end of 2024, and its intention to pursue clinical development of deucrictibant in a newly named indication, acquired angioedema (AAE).
Pharvaris Presents Deucrictibant Long-Term Extension Data for both the Prophylactic and On-Demand Treatment of HAE at the Bradykinin Symposium 2024
On September 5, 2024, the Company issued a press release titled “Pharvaris Presents Deucrictibant Long-Term Extension Data for both the Prophylactic and On-Demand Treatment of HAE at the Bradykinin Symposium 2024” highlighting certain presentations to be made on deucrictibant as a prophylactic and on-demand treatment of HAE attacks at the 7th Bradykinin Symposium, being held in Berlin from September 5-6, 2024, summarized below.
CHAPTER-1 (NCT05047185) is a two-part Phase 2 study evaluating the efficacy and safety of deucrictibant for long-term prophylaxis of HAE attacks. Positive top-line data from the double-blind, randomized, placebo-controlled portion (part 1) of the CHAPTER-1 study were announced in December 2023. Further exploration of health-related quality of life (HRQoL), disease control, and treatment satisfaction data will be presented by Dr. Markus Magerl in an oral presentation and will show that 90% of participants receiving deucrictibant (N=20) reported well-controlled HAE symptoms at week 12 compared to 37.5% of participants receiving placebo (N=8) as measured by the Angioedema Control Test (AECT). Deucrictibant treated participants experienced greater efficacy and global satisfaction compared to placebo, while experiencing placebo-like side effects.
Upon completing part 1 of CHAPTER-1, all eligible participants (N=30) enrolled into the ongoing open-label extension (part 2) during which they received deucrictibant 40 mg/day with a mean treatment duration of 12.83 months. The current analysis (cutoff date: June 10, 2024) will be presented by Dr. Riedl in a poster presentation and show that deucrictibant was well-tolerated, with no safety signals observed. Efficacy analyses show:
•Deucrictibant reduced the attack rate in the open-label extension by 93.0% compared to the part 1 study baseline.
•The reduction in occurrence of moderate and severe attacks and of attacks treated with on-demand medication remained low in the open-label extension.
RAPIDe-2 (NCT05396105) isan ongoing two-part Phase 2/3 extension study, evaluating long-term safety and efficacy of orally administered deucrictibant for the on-demand treatment of HAE attacks. The safety analysis (cutoff date: June 10, 2024) includes a total of 337 attacks and shows that deucrictibant was well-tolerated for all studied doses with no new safety signals observed. The efficacy analysis (cutoff date: March 1, 2024) includes a total of 265 attacks and shows:
•Median time to onset of symptom relief was 1.1 hours with 98.5% of attacks achieving onset of symptom relief by 12 hours (PGI-C).
•Median time to reduction in attack severity was 2.6 hours with 97.7% of attacks achieving reduction in attack severity by 12 hours (Patient Global Impression of Severity [PGI-S]).
•Median time to complete attack resolution was 11.5 hours with 85.8% of attacks achieving complete attack resolution within 24 hours and 90.2% of those attacks requiring only one dose of deucrictibant (PGI-S).
Pharvaris Presents Data at the Bradykinin Symposium 2024
On September 5, 2024, the Company issued a press release titled “Pharvaris Presents Data at the Bradykinin Symposium 2024” which announced a summary of data being presented at the ongoing 7th Bradykinin Symposium. The presentations are listed below:
•Long-Term Safety and Efficacy of Oral Deucrictibant for HAE Prophylaxis, a poster presentation by Marc A. Riedl, M.D., M.A.
•Treatment of HAE Attacks with Oral Deucrictibant: RAPIDe-2 Extension Results, a poster presentation by Emel Aygören-Pürsün, M.D.
•Prophylactic Treatment with Deucrictibant Improves HAE Disease Control and HRQoL, an oral presentation by Markus Magerl, M.D.
•Deucrictibant vs. Standard of Care in HAE: Propensity Score-Matched Analysis, a poster presentation by Marc A. Riedl, M.D., M.A.
•Prophylaxis of Hereditary Angioedema Attacks with Oral Deucrictibant: CHAPTER-1 Results, a poster presentation by Emel Aygören-Pürsün, M.D.
•Clinical Trials Conformity with AURORA COS: a systematic literature review, a poster presentation by Remy Petersen, M.D., Ph.D.
•Bradykinin Challenge Model in Humanized Bradykinin B2 receptor Transgenic Rat, an oral presentation by Jolanta Skarbaliene, Ph.D.
•Deucrictibant inhibits carrageenan-induced edema in bradykinin B2 receptor transgenic rat, a poster presentation by Anne Lesage, Ph.D.
•The bradykinin challenge model translates across rat, monkey and human, a poster presentation by Juan Bravo, Ph.D.
•A novel kinin biomarker assay for characterization of bradykinin-mediated disorders, a poster presentation by Evangelia Pardali, Ph.D.
•A HMWK capillary immunoblotting assay to characterize bradykinin-mediated disorders, a poster presentation by Evangelia Pardali, Ph.D.
On September 5, 2024, the Company made available a corporate presentation on its website.
Except as stated herein, this Report on Form 6-K shall be deemed to be incorporated by reference into the registration statements on Form F-3 (Registration Numbers 333-278650, 333-277705 and 333-273757) and Form S-8 (Registration Number 333-252897) of Pharvaris N.V. and to be a part thereof from the date on which this report is filed, to the extent not superseded by documents or reports subsequently filed or furnished.
Copies of the press releases and corporate presentation are attached hereto as Exhibits 99.1, 99.2, 99.3 and 99.4. Exhibits 99.1, 99.2, 99.3 and 99.4 to this Report on Form 6-K shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended or the Exchange Act.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
PHARVARIS N.V. |
|
|
Date: September 5, 2024 |
By: |
/s/ Berndt Modig |
|
Name: |
Berndt Modig |
|
Title: |
Chief Executive Officer |
EXHIBIT INDEX

Exhibit 99.1
Pharvaris Presents Data at the Bradykinin Symposium 2024
ZUG, Switzerland, September 5, 2024 -- Pharvaris (Nasdaq: PHVS), a late-stage biopharmaceutical company developing novel, oral bradykinin B2 receptor antagonists to prevent and treat hereditary angioedema (HAE) attacks, today announced a summary of data being presented at the ongoing 7th Bradykinin Symposium. Details of the presentations are outlined below:
Long-Term Safety and Efficacy of Oral Deucrictibant for HAE Prophylaxis, a poster presentation by Marc A. Riedl, M.D., M.S. In the current analysis of the ongoing open-label extension of the CHAPTER-1 Phase 2 study, deucrictibant 40 mg/day was well-tolerated, with no new safety signals observed. The results presented provide evidence of the long-term safety and efficacy of deucrictibant for the prevention of HAE attacks and support further development of deucrictibant as a potential prophylactic therapy for HAE. Results of this analysis provide support that:
•Continuing deucrictibant treatment sustained the early-onset attack reduction seen in the randomized, placebo-controlled portion of the trial, with a median attack rate of zero for every month for over a year in the open-label part of the study
•On average, less than one attack per year per participant was treated with on-demand medication
Treatment of HAE Attacks with Oral Deucrictibant: RAPIDe-2 Extension Results, a poster presentation by Emel Aygören-Pürsün, M.D. In the current analysis of the ongoing RAPIDe-2 Phase 2/3 extension study, deucrictibant immediate release capsule was well-tolerated for all studied doses with no new safety signals observed. Results from the ongoing RAPIDe-2 extension are consistent with the randomized, placebo-controlled RAPIDe-1 Phase 2 study and provide evidence regarding the long-term safety and efficacy of deucrictibant IR capsule for repeat treatment of HAE attacks. Outcome analyses showed:
•Median time to onset of symptom relief as measured by the Patient Global Impression of Change (PGI-C) was 1.1 hours, with 98.5% of attacks achieving onset of symptom relief by 12 hours
•Median time to reduction in attack severity as measured by the Patient Global Impression of Severity (PGI-S) was 2.6 hours, with 97.7% of attacks achieving reduction in attack severity by 12 hours
•Median time to complete attack resolution as measure by PGI-S was 11.5 hours, with 85.8% of attacks achieving complete attack resolution within 24 hours
•Overall, 86.0% of attacks were treated with a single dose of deucrictibant immediate-release capsule
Prophylactic Treatment with Deucrictibant Improves HAE Disease Control and HRQoL, an oral presentation by Markus Magerl, M.D. In the randomized, placebo-controlled part of CHAPTER-1, a Phase 2 clinical study of deucrictibant for the prophylactic treatment of HAE attacks, health-related quality of life was evaluated using several measures. In the study, it was demonstrated that deucrictibant treatment led to improvements in disease control versus placebo, with 90% of participants in the deucrictibant-groups demonstrating well-controlled HAE at week 12. Presentation details included:
•Deucrictibant improved Angioedema Quality of Life Questionnaire (AE-QoL) scores, particularly in “functioning” and “fear/shame” domains compared to placebo
•Deucrictibant-treated participants reported greater satisfaction than those treated with placebo with regards to effectiveness and the domain of global satisfaction, and a comparable satisfaction for side effects, as measured by Treatment Satisfaction Questionnaire for Medication (TSQM)
Deucrictibant vs. Standard of Care in HAE: Propensity Score-Matched Analysis, a poster presentation by Marc A. Riedl, M.D., M.S. A propensity score-matched comparison of clinical outcomes between a subgroup of attacks (N=73) from the RAPIDe-2 study and a subgroup of attacks (N=73) from an observational real-world study treated with standard of care, the outcomes were more favorable for the attacks treated with deucrictibant on PGI-C- and PGI-S-based assessments. Deucrictibant had a shorter (1.07 hours) median time to onset of symptom relief as measured by PGI-C “a little better” compared to standard of care (2.38 hours).
Cardiovascular safety of repeated oral administration of the B2-receptor antagonist deucrictibant, a poster presentation by Nieves Crespo, Ph.D. In chronic nonclinical safety studies of deucrictibant in non-human primates, no evident effects on cardiac electrophysiology, morphology and hemodynamic parameters were observed. Deucrictibant has showed no evident effects on cardiac electrophysiology and hemodynamic parameters in clinical studies in humans to date, following prophylactic treatment up to 12 weeks of administration in the randomized, placebo-controlled part of the Phase 2 CHAPTER-1 clinical study and up to one year in the ongoing open-label extension (OLE) part.
Prophylaxis of Hereditary Angioedema Attacks with Oral Deucrictibant: CHAPTER-1 Results, a poster presentation by Emel Aygören-Pürsün, M.D. The CHAPTER-1 study demonstrated that deucrictibant may significantly reduce the occurrence of HAE attacks, and clinically meaningful
reduction in occurrence of moderate and severe HAE attacks, as well as HAE attacks treated with on-demand medication, was observed. CHAPTER-1 results provide evidence of the efficacy and safety of deucrictibant for the prevention of HAE attacks and support its further development as a potential prophylactic therapy for HAE.
Clinical Trials Conformity with AURORA COS: a systematic literature review, a poster presentation by Remy S. Petersen, M.D., Ph.D. Conforming to a core outcome set (COS) across various study designs, such as the COS recommended for HAE clinical studies by the Panel of Experts participating in the AURORA Project, may homogenize the use of specific outcomes for clinical studies and support future indirect comparisons among interventions. The design of the RAPIDe-3 Phase 3 study of deucrictibant immediate-release capsule for the on-demand treatment of HAE attacks fully conforms with the AURORA COS based on its prespecified endpoints.
Bradykinin Challenge Model in Humanized Bradykinin B2 receptor Transgenic Rat, an oral presentation by Jolanta Skarbaliene, Ph.D. The bradykinin (BK) challenge model is a tool to assess pharmacokinetic and pharmacodynamic activity of bradykinin B2 receptor antagonists. A BK challenge model was successfully developed in humanized bradykinin B2 receptor transgenic rats that are pharmacologically responsive to bradykinin B2 receptor antagonists. The BK challenge model in humanized bradykinin B2 receptor transgenic rats can offer a valuable, easy to manage, and cost-effective tool for efficacy studies compared to those involving non-human primates.
Deucrictibant inhibits carrageenan-induced edema in bradykinin B2 receptor transgenic rat, a poster presentation by Anne Lesage, Ph.D. A humanized bradykinin B2 receptor transgenic rat model was used to address the challenge of deucrictibant species selectivity in experimental models. Oral deucrictibant inhibited carrageenan-induced paw edema in humanized bradykinin B2 receptor transgenic rats.
The bradykinin challenge model translates across rat, monkey and human, a poster presentation by Juan Bravo, Ph.D. The pharmacokinetics (PK) and pharmacodynamics (PD) of icatibant were analyzed from BK challenge studies in humanized bradykinin B2 receptor transgenic rats, non-human primates, and healthy volunteers. Analyses across species showed similar responses, demonstrating that the BK challenge model in transgenic rats and non-human primates may be predictive of PK/PD outcomes in humans.
A novel kinin biomarker assay for characterization of bradykinin-mediated disorders, a poster presentation by Evangelia Pardali, Ph.D. BK is involved in various physiological and pathological processes, including angioedema (AE). Differentiating BK-mediated from histamine-mediated AE and assessing the role of BK in the pathogenesis of other conditions by measuring kinin peptides remains a challenge due to their proteolytic instability and limitations of current analytical assays. A kinin biomarker assay was established and qualified, which could become a key tool for identifying, studying, and managing BK-mediated diseases.
A HMWK capillary immunoblotting assay to characterize bradykinin-mediated disorders, a poster presentation by Evangelia Pardali, Ph.D. Activation of the plasma kallikrein-kinin system (KKS) can result in cleavage of high molecular weight kininogen (HMWK) and production of vasodilatory kinins, such as BK. A HMWK immunoblotting assay was established and qualified to reliably measure KKS biomarkers in human plasma and could become a key tool for identifying, studying, and managing BK-mediated diseases.
The presentation slides and posters are available on the Investors section of the Pharvaris website at: https://ir.pharvaris.com/news-events/events-presentations.
About Deucrictibant
Deucrictibant is a novel, potent, oral small-molecule bradykinin B2 receptor antagonist. By inhibiting bradykinin signaling through the bradykinin B2 receptor, deucrictibant has the potential to prevent the occurrence of HAE attacks and to treat the manifestations of attacks if they occur. Based on its chemical properties, Pharvaris is developing two formulations of deucrictibant for oral administration: an extended-release tablet to enable sustained absorption and efficacy for prophylactic treatment, and an immediate-release capsule to enable rapid onset of activity for on-demand treatment.
About Pharvaris
Pharvaris is a late-stage biopharmaceutical company developing novel, oral bradykinin B2 receptor antagonists to prevent and treat HAE attacks. By directly pursuing this clinically proven therapeutic target with novel small molecules, the Pharvaris team aspires to offer people with all types of bradykinin-mediated angioedema effective, well-tolerated, and easy-to-administer alternatives to treat attacks, both prophylactically and on-demand . With positive data in both Phase 2 prophylaxis and on-demand studies in HAE, Pharvaris is encouraged to further develop deucrictibant. Pharvaris is currently enrolling a pivotal Phase 3 study for the on-demand treatment of HAE attacks and plans to initiate a pivotal Phase 3
study of deucrictibant for the prevention of HAE attacks in the coming months. For more information, visit https://pharvaris.com/.
Forward-Looking Statements
This press release contains certain forward-looking statements that involve substantial risks and uncertainties. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements relating to our future plans, studies and trials, and any statements containing the words “believe,” “anticipate,” “expect,” “estimate,” “may,” “could,” “should,” “would,” “will,” “intend” and similar expressions. These forward-looking statements are based on management’s current expectations, are neither promises nor guarantees, and involve known and unknown risks, uncertainties and other important factors that may cause Pharvaris’ actual results, performance or achievements to be materially different from its expectations expressed or implied by the forward-looking statements. Such risks include but are not limited to the following: uncertainty in the outcome of our interactions with regulatory authorities, including the FDA; the expected timing, progress, or success of our clinical development programs, especially for deucrictibant immediate-release capsules and deucrictibant extended-release tablets, which are in late-stage global clinical trials; our ability to replicate the efficacy and safety demonstrated in the RAPIDe-1, RAPIDe-2, and CHAPTER-1 Phase 2 studies in ongoing and future nonclinical studies and clinical trials; risks arising from epidemic diseases, such as the COVID-19 pandemic, which may adversely impact our business, nonclinical studies, and clinical trials; the outcome and timing of regulatory approvals; the value of our ordinary shares; the timing, costs and other limitations involved in obtaining regulatory approval for our product candidates, or any other product candidate that we may develop in the future; our ability to establish commercial capabilities or enter into agreements with third parties to market, sell, and distribute our product candidates; our ability to compete in the pharmaceutical industry, including with respect to existing therapies, emerging potentially competitive therapies and with competitive generic products; our ability to market, commercialize and achieve market acceptance for our product candidates; our ability to raise capital when needed and on acceptable terms; regulatory developments in the United States, the European Union and other jurisdictions; our ability to protect our intellectual property and know-how and operate our business without infringing the intellectual property rights or regulatory exclusivity of others; our ability to manage negative consequences from changes in applicable laws and regulations, including tax laws, our ability to successfully remediate the material weaknesses in our internal control over financial reporting and to maintain an effective system of internal control over financial reporting; changes and uncertainty in general market, political and economic conditions, including as a result of inflation and the current conflict between Russia and Ukraine and the Hamas
attack against Israel and the ensuing war; and the other factors described under the headings “Cautionary Statement Regarding Forward-Looking Statements” and “Item 3. Key Information—D. Risk Factors” in our Annual Report on Form 20-F and other periodic filings with the U.S. Securities and Exchange Commission. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. While Pharvaris may elect to update such forward-looking statements at some point in the future, Pharvaris disclaims any obligation to do so, even if subsequent events cause its views to change. These forward-looking statements should not be relied upon as representing Pharvaris’ views as of any date subsequent to the date of this press release.
Contact
Maggie Beller
Executive Director, Head of Corporate and Investor Communications
maggie.beller@pharvaris.com

Exhibit 99.2
Pharvaris Presents Deucrictibant Long-Term Extension Data for Both the Prophylactic and On-Demand Treatment of HAE at the Bradykinin Symposium 2024
•Extension data confirm the observed safety and tolerability profile from Phase 2 studies and further support the potential for deucrictibant to become a preferred therapy for the management of HAE
•Long-term prophylaxis extension data of deucrictibant shows attack reduction is maintained for over one year; open-label extension participants experienced a 93% reduction in attacks compared to baseline
•Long-term on-demand extension data of deucrictibant immediate-release capsule shows median onset of symptom relief in ~1.1 hours, with 85.8% of attacks resolving completely within 24 hours
ZUG, Switzerland, September 5, 2024 -- Pharvaris (Nasdaq: PHVS), a late-stage biopharmaceutical company developing novel, oral bradykinin B2 receptor antagonists to prevent and treat hereditary angioedema (HAE) attacks, is highlighting the differentiated profile of deucrictibant as a prophylactic and on-demand treatment of HAE attacks at the Bradykinin Symposium 2024, being held in Berlin from September 5-6, 2024. A summary of the data being presented at the congress can be found here.
“Based on a snapshot analysis, treatment with deucrictibant led to a 93% reduction in attack rate compared to study baseline, a median attack rate of zero for every month, and a mean proportion of attack-free days of 99% after more than a year of mean duration of treatment in a prophylactic extension study. Together with the improvements in disease control and health-related quality of life observed in the randomized, placebo-controlled part of the CHAPTER-1 study, these data underscore the potential of deucrictibant to be an effective and well-tolerated prophylactic agent in the treatment of HAE,” said Peng Lu, M.D., Ph.D., Chief Medical Officer of Pharvaris. “Long-term extension data of deucrictibant in the on-demand setting similarly confirm its potential to become a preferred option for the treatment of HAE attacks with a median onset of symptom relief of 1.1 hours, as measured by Patient Global Impression of Change (PGI-C) and median complete resolution of 11.5 hours, as measured by Patient Global Impression of Severity (PGI-S). The rapid onset of symptom relief reported in RAPIDe-2 and the results of a propensity score-matched analysis favoring deucrictibant over standard of care provide confidence in our ability to differentiate deucrictibant in the on-demand HAE space. Lastly, the safety and tolerability profile of deucrictibant has been reaffirmed in multiple nonclinical and clinical studies.”
Marc A. Riedl, M.D., M.S., Professor of Medicine, Clinical Director of the U.S. Hereditary Angioedema Association (HAEA) Angioedema Center at the University of California San Diego (UCSD), Clinical Service Chief for Allergy/Immunology at UCSD, added, “The goal of HAE management is for affected individuals to live a normal life, ensuring they can engage in all work, school, family, and leisure activities as desired without limitation from angioedema symptoms. Therapies that offer improved efficacy, tolerability, and convenience have the potential to normalize the lives of people living with HAE. These long-term extension and health-related quality of life data, together with the Phase 2 clinical trial data, provide evidence of the benefits of deucrictibant as a potential treatment for HAE, and highlight the importance of additional data from late-stage clinical development of deucrictibant in both treatment settings.”
Prophylactic Program
CHAPTER-1 (NCT05047185) is a two-part Phase 2 study evaluating the efficacy and safety of deucrictibant for long-term prophylaxis of HAE attacks. Positive top-line data from the double-blind, randomized, placebo-controlled portion (part 1) of the CHAPTER-1 study were announced in December 2023. Further exploration of disease control, health-related quality of life (HRQoL), and treatment satisfaction data will be presented by Dr. Markus Magerl in an oral presentation and show that 90% of participants receiving deucrictibant (N=20) reported well-controlled HAE at week 12 compared to 37.5% of participants receiving placebo (N=8) as measured by the Angioedema Control Test (AECT). Deucrictibant-treated participants reported greater satisfaction than those treated with placebo with regards to effectiveness and the domain of global satisfaction, and a comparable satisfaction for side effects.
All eligible participants completing part 1 of CHAPTER-1 (N=30) enrolled into the ongoing open-label extension (part 2) during which they have received deucrictibant 40 mg/day with a mean treatment duration in the extension of 12.83 months. The current analysis (cutoff date: June 10, 2024) will be presented by Dr. Riedl in a poster presentation and show that deucrictibant was well-tolerated, with no safety signals observed. Efficacy analyses show:
•Deucrictibant reduced the attack rate in the open-label extension by 93.0% compared to the part 1 study baseline
•The occurrence of “moderate and severe” attacks and of attacks treated with on-demand medication remained low in the open-label extension
On-Demand Program
Dr. Emel Aygören-Pürsün will present a poster on RAPIDe-2 (NCT05396105), an ongoing two-part Phase 2/3 extension study, evaluating long-term safety and efficacy of orally administered deucrictibant immediate-release capsule for the on-demand treatment of HAE attacks. The safety analysis (cutoff date: June 10, 2024) includes a total of 337 attacks and shows that deucrictibant was well-tolerated for all studied doses with no new safety signals observed. The efficacy analysis (cutoff date: March 1, 2024) includes a total of 265 attacks and shows:
•Median time to onset of symptom relief was 1.1(PGI-C) hours with 98.5% of attacks achieving onset of symptom relief by 12 hours
•Median time to reduction in attack severity was 2.6 hours (PGI-S) with 97.7% of attacks achieving reduction in attack severity by 12 hours
•Median time to complete attack resolution was 11.5 hours with 85.8% of attacks achieving complete attack resolution within 24 hours (PGI-S) and 90.2% of these attacks requiring a single dose of deucrictibant
RAPIDe-2 data were used to conduct a comparison of deucrictibant immediate-release capsule to standard of care on-demand therapy in a propensity-score matched analysis. The analysis to be presented by Dr. Riedl in a poster presentation, indicates that PGI-C- and PGI-S-based outcomes were more favorable for the attacks treated with deucrictibant in RAPIDe-2 study than for the attacks treated with standard of care in an observational study.
Safety
Dr. Nieves Crespo will present a poster on an assessment of the cardiovascular safety of deucrictibant after repeated dosing which shows no evidence of any impact on cardiovascular parameters in nonclinical studies in non-human primates, including a 3-month and chronic study, nor in clinical studies to date, following prophylactic treatment up to 12 weeks of administration in the randomized, placebo-controlled part 1 of CHAPTER-1 clinical study and up to one year in its ongoing open-label extension.
The presentation slides and posters are available on the Investors section of the Pharvaris website at: https://ir.pharvaris.com/news-events/events-presentations.
About Deucrictibant
Deucrictibant is a novel, potent, oral small-molecule bradykinin B2 receptor antagonist. By inhibiting bradykinin signaling through the bradykinin B2 receptor, deucrictibant has the potential to prevent the
occurrence of HAE attacks and to treat the manifestations of an attack if they occur. Based on its chemical properties, Pharvaris is developing two formulations of deucrictibant for oral administration: an extended-release tablet to enable sustained absorption and efficacy for prophylactic treatment, and an immediate-release capsule to enable rapid onset of activity for on-demand treatment.
About Pharvaris
Pharvaris is a late-stage biopharmaceutical company developing novel, oral bradykinin B2 receptor antagonists to prevent and treat HAE attacks. By directly pursuing this clinically proven therapeutic target with novel small molecules, the Pharvaris team aspires to offer people with all types of bradykinin-mediated angioedema effective, well-tolerated, and easy-to-administer alternatives to treat attacks, both prophylactically and on-demand . With positive data in both Phase 2 prophylaxis and on-demand studies in HAE, Pharvaris is encouraged to further develop deucrictibant. Pharvaris is currently enrolling a pivotal Phase 3 study for the on-demand treatment of HAE attacks and plans to initiate a pivotal Phase 3 study of deucrictibant for the prevention of HAE attacks in the coming months. For more information, visit https://pharvaris.com/.
Forward-Looking Statements
This press release contains certain forward-looking statements that involve substantial risks and uncertainties. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements relating to our future plans, studies and trials, and any statements containing the words “believe,” “anticipate,” “expect,” “estimate,” “may,” “could,” “should,” “would,” “will,” “intend” and similar expressions. These forward-looking statements are based on management’s current expectations, are neither promises nor guarantees, and involve known and unknown risks, uncertainties and other important factors that may cause Pharvaris’ actual results, performance or achievements to be materially different from its expectations expressed or implied by the forward-looking statements. Such risks include but are not limited to the following: uncertainty in the outcome of our interactions with regulatory authorities, including the FDA; the expected timing, progress, or success of our clinical development programs, especially for deucrictibant immediate-release capsules and deucrictibant extended-release tablets, which are in late-stage global clinical trials; our ability to replicate the efficacy and safety demonstrated in the RAPIDe-1, RAPIDe-2, and CHAPTER-1 Phase 2 studies in ongoing and future nonclinical studies and clinical trials; risks arising from epidemic diseases, such as the COVID-19 pandemic, which may adversely impact our business, nonclinical studies, and clinical trials; the outcome and timing of regulatory approvals; the value of our ordinary shares; the timing, costs and other limitations involved in obtaining regulatory approval
for our product candidates, or any other product candidate that we may develop in the future; our ability to establish commercial capabilities or enter into agreements with third parties to market, sell, and distribute our product candidates; our ability to compete in the pharmaceutical industry, including with respect to existing therapies, emerging potentially competitive therapies and with competitive generic products; our ability to market, commercialize and achieve market acceptance for our product candidates; our ability to raise capital when needed and on acceptable terms; regulatory developments in the United States, the European Union and other jurisdictions; our ability to protect our intellectual property and know-how and operate our business without infringing the intellectual property rights or regulatory exclusivity of others; our ability to manage negative consequences from changes in applicable laws and regulations, including tax laws, our ability to successfully remediate the material weaknesses in our internal control over financial reporting and to maintain an effective system of internal control over financial reporting; changes and uncertainty in general market, political and economic conditions, including as a result of inflation and the current conflict between Russia and Ukraine and the Hamas attack against Israel and the ensuing war; and the other factors described under the headings “Cautionary Statement Regarding Forward-Looking Statements” and “Item 3. Key Information—D. Risk Factors” in our Annual Report on Form 20-F and other periodic filings with the U.S. Securities and Exchange Commission. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. While Pharvaris may elect to update such forward-looking statements at some point in the future, Pharvaris disclaims any obligation to do so, even if subsequent events cause its views to change. These forward-looking statements should not be relied upon as representing Pharvaris’ views as of any date subsequent to the date of this press release.
Contact
Maggie Beller
Executive Director, Head of Corporate and Investor Communications
maggie.beller@pharvaris.com

Exhibit 99.3
Pharvaris Provides Business Update and Expands Development Program for Deucrictibant
•CHAPTER-3, the global pivotal Phase 3 clinical study of deucrictibant for the prophylactic treatment for HAE using once-daily extended-release tablet, is expected to initiate by YE2024
•Differentiated deucrictibant profile, including long-term extension results, to be highlighted in clinical, real-world, nonclinical, and discovery data presentations at the 2024 Bradykinin Symposium
•Pharvaris intends to pursue clinical development in acquired angioedema as a newly named indication
•Pharvaris to host a conference call today at 8:00 a.m. ET
ZUG, Switzerland, September 5, 2024 -- Pharvaris (Nasdaq: PHVS), a late-stage biopharmaceutical company developing novel, oral bradykinin B2 receptor antagonists to prevent and treat hereditary angioedema (HAE) attacks, today announced the planned initiation of CHAPTER-3, the pivotal Phase 3 study of deucrictibant extended-release tablets for the prophylactic treatment of HAE; announced its intention to pursue clinical development of deucrictibant in a newly named indication, acquired angioedema due to C1-inhibitor deficiency (AAE-C1INH); and presented a robust data set highlighting the differentiating characteristics of deucrictibant.
“Given the totality of data for deucrictibant, now bolstered by new data from ongoing long-term extension studies showing tolerability and efficacy in both prophylaxis and on-demand treatment, we believe deucrictibant has the potential to become a preferred therapy for the management of HAE,” said Berndt Modig, Chief Executive Officer at Pharvaris. “We remain focused on the efficient execution of our clinical studies, with the CHAPTER-3 study expected to initiate by the end of the year while RAPIDe-3 is progressing as planned. Pharvaris has the expertise to expand deucrictibant beyond HAE to other bradykinin-mediated-disease—such as AAE-C1INH—and we are excited to explore the potential for deucrictibant to meet a currently unaddressed medical need.”
CHAPTER-3, a global, pivotal Phase 3 study of deucrictibant extended-release tablet for the prophylactic treatment of HAE attacks, is expected to initiate by year end 2024.
Startup activities are on track to initiate CHAPTER-3 by the end of 2024. CHAPTER-3 will assess the efficacy and safety of once-daily dosing of the extended-release tablet formulation of deucrictibant, which is designed to provide sustained protection from HAE attacks by maintaining plasma exposure above
therapeutic level for over 24 hours and achieving pharmacokinetic steady state in approximately two to three days.
Stefan Abele, Ph.D., Chief Technical Operations Officer of Pharvaris, commented, “Pharvaris’ supply chain and CMC teams have been working diligently to ensure timely delivery of deucrictibant extended-release tablets in the commercial formulation to our Phase 3 clinical sites. The use of deucrictibant extended-release tablets in the CHAPTER-3 Phase 3 study enables us to evaluate deucrictibant’s ability to address the need for improvements in quality-of-life that people living with HAE want and deserve: a therapy providing injectable-like efficacy, from the first day of therapy, with a favorable tolerability and the convenience of once-daily oral administration.”
Pharvaris intends to pursue clinical development of deucrictibant in AAE-C1INH following publication of compelling data from an investigator-initiated trial. Data in the Journal of Allergy and Clinical Immunology in July 2024 explored the potential for deucrictibant to address the unmet medical need for well-tolerated and effective therapies for the prophylactic and on-demand treatment of AAE-C1INH. A randomized, double-blind, placebo-controlled study was conducted by Investigators at the Amsterdam University Medical Center (Amsterdam UMC). Three people living with AAE-C1INH were enrolled; the individual mean monthly attack rates were 2.0, 0.6, and 1.0 during the placebo period and 0.0 across all participants during treatment with deucrictibant. There were no severe adverse events and one self-limiting treatment-emergent adverse event (abdominal pain).
Remy Petersen, M.D., at Amsterdam UMC, stated, “There is an unmet need for therapies approved specifically for the treatment of AAE-C1INH. At Amsterdam UMC, we were pleased to confirm our hypothesis that a bradykinin B2 receptor antagonist, such as deucrictibant, has the potential to successfully prevent and treat AAE-C1INH. We look forward to continuing our collaboration with Pharvaris in the clinical development of deucrictibant for AAE-C1INH to further demonstrate the therapeutic benefit for those living with bradykinin-mediated angioedema.”
Differentiated clinical profile of deucrictibant presented at the Bradykinin Symposium.
A snapshot of long-term extension data from the ongoing prophylactic (CHAPTER-1 part 2: NCT05047185) and on-demand (RAPIDe-2: NCT05396105) extension studies provide evidence of the sustained product profile of deucrictibant in both HAE treatment settings. Additional information can be found in the detailed data press release and in the complete presentation summary. The presentation slides and posters are available on the Investors section of the Pharvaris website.
Upcoming Event
CIIC Fall 2024 Conference. Dallas, TX, September 13-14, 2024. Two abstracts have been accepted for e-Poster presentation. Details are as follows:
•Title: Long-Term Efficacy and Safety of Oral Deucrictibant, a Bradykinin B2 Receptor Antagonist, in Treatment of Hereditary Angioedema Attacks: Results of the RAPIDe-2 Extension Study
Presenter: Joshua S. Jacobs, M.D.
Format: ePoster
•Title: Long-Term Safety and Efficacy of Prophylactic Oral Deucrictibant, a Bradykinin B2 Receptor Antagonist, in Hereditary Angioedema: Results of the CHAPTER-1 Open Label Extension Study
Format: Looped e-Poster Display Board
Conference Call and Webcast
Pharvaris will host a live conference call and webcast today to discuss these updates and data in greater detail at 8:00 a.m. EDT via a live webcast; presentation slides may be accessed on the “Events and Presentations” page of the Pharvaris investor relations website. Participants interested in asking a question during the Q&A may do so in the live conference call. An archived replay will also be available on the website for 90 days following the event.
About Deucrictibant
Deucrictibant is a novel, potent, oral small-molecule bradykinin B2 receptor antagonist. By inhibiting bradykinin signaling through the bradykinin B2 receptor, deucrictibant has the potential to prevent the occurrence of HAE attacks and to treat the manifestations of attacks if they occur. Based on its chemical properties, Pharvaris is developing two formulations of deucrictibant for oral administration: an extended-release tablet to enable sustained absorption and efficacy for prophylactic treatment, and an immediate-release capsule to enable rapid onset of activity for on-demand treatment.
About Pharvaris
Pharvaris is a late-stage biopharmaceutical company developing novel, oral bradykinin B2 receptor antagonists to prevent and treat HAE attacks. By directly pursuing this clinically proven therapeutic target with novel small molecules, the Pharvaris team aspires to offer people with all types of bradykinin-mediated angioedema effective, well-tolerated, and easy-to-administer alternatives to treat attacks, both
prophylactically and on-demand . With positive data in both Phase 2 prophylaxis and on-demand studies in HAE, Pharvaris is encouraged to further develop deucrictibant. Pharvaris is currently enrolling a pivotal Phase 3 study for the on-demand treatment of HAE attacks and plans to initiate a pivotal Phase 3 study of deucrictibant for the prevention of HAE attacks in the coming months. For more information, visit https://pharvaris.com/.
Forward-Looking Statements
This press release contains certain forward-looking statements that involve substantial risks and uncertainties. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements relating to our future plans, studies and trials, and any statements containing the words “believe,” “anticipate,” “expect,” “estimate,” “may,” “could,” “should,” “would,” “will,” “intend” and similar expressions. These forward-looking statements are based on management’s current expectations, are neither promises nor guarantees, and involve known and unknown risks, uncertainties and other important factors that may cause Pharvaris’ actual results, performance or achievements to be materially different from its expectations expressed or implied by the forward-looking statements. Such risks include but are not limited to the following: uncertainty in the outcome of our interactions with regulatory authorities, including the FDA; the expected timing, progress, or success of our clinical development programs, especially for deucrictibant immediate-release capsules and deucrictibant extended-release tablets, which are in late-stage global clinical trials; our ability to replicate the efficacy and safety demonstrated in the RAPIDe-1, RAPIDe-2, and CHAPTER-1 Phase 2 studies in ongoing and future nonclinical studies and clinical trials; risks arising from epidemic diseases, such as the COVID-19 pandemic, which may adversely impact our business, nonclinical studies, and clinical trials; the outcome and timing of regulatory approvals; the value of our ordinary shares; the timing, costs and other limitations involved in obtaining regulatory approval for our product candidates, or any other product candidate that we may develop in the future; our ability to establish commercial capabilities or enter into agreements with third parties to market, sell, and distribute our product candidates; our ability to compete in the pharmaceutical industry, including with respect to existing therapies, emerging potentially competitive therapies and with competitive generic products; our ability to market, commercialize and achieve market acceptance for our product candidates; our ability to raise capital when needed and on acceptable terms; regulatory developments in the United States, the European Union and other jurisdictions; our ability to protect our intellectual property and know-how and operate our business without infringing the intellectual property rights or regulatory exclusivity of others; our ability to manage negative consequences from changes in applicable laws and regulations, including tax laws, our ability to successfully remediate the material weaknesses in our
internal control over financial reporting and to maintain an effective system of internal control over financial reporting; changes and uncertainty in general market, political and economic conditions, including as a result of inflation and the current conflict between Russia and Ukraine and the Hamas attack against Israel and the ensuing war; and the other factors described under the headings “Cautionary Statement Regarding Forward-Looking Statements” and “Item 3. Key Information—D. Risk Factors” in our Annual Report on Form 20-F and other periodic filings with the U.S. Securities and Exchange Commission. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. While Pharvaris may elect to update such forward-looking statements at some point in the future, Pharvaris disclaims any obligation to do so, even if subsequent events cause its views to change. These forward-looking statements should not be relied upon as representing Pharvaris’ views as of any date subsequent to the date of this press release.
Contact
Maggie Beller
Executive Director, Head of Corporate and Investor Communications
maggie.beller@pharvaris.com

Pioneering science for patient choice Corporate Presentation September 2024 Exhibit 99.4

Disclaimer This Presentation contains certain “forward‐looking statements” within the meaning of the federal securities laws that involve substantial risks and uncertainties. All statements contained in this Presentation that do not relate to matters of historical fact should be considered forward-looking statements including, without limitation, statements containing the words “believe,” “anticipate,” “expect,” “estimate,” “may,” “could,” “should,” “would,” “will,” “intend” and similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Such forward-looking statements involve unknown risks, uncertainties and other factors which may cause our actual results, financial condition, performance or achievements, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Factors that might cause such a difference include, but are not limited to, uncertainty in the outcome of our interactions with regulatory authorities, including the FDA, the expected timing, progress, or success of our clinical development programs, especially for deucrictibant, which are in late-stage global clinical trials, our ability to replicate the efficacy and safety demonstrated in the RAPIDe-1, RAPIDe-2, and CHAPTER-1 Phase 2 studies in ongoing and future nonclinical studies and clinical trials, risks arising from epidemic diseases such as the COVID-19 pandemic, which may adversely impact our business, nonclinical studies, and clinical trials, the outcome and timing of regulatory approvals, the value of our ordinary shares, the timing, costs and other limitations involved in obtaining regulatory approval for our product candidates, or any other product candidate that we may develop in the future, our ability to establish commercial capabilities or enter into agreements with third parties to market, sell, and distribute our product candidates, our ability to compete in the pharmaceutical industry, including with respect to existing therapies, emerging potentially competitive therapies and with competitive generic products, our ability to market, commercialize and achieve market acceptance for our product candidates, our ability to raise capital when needed and on acceptable terms, regulatory developments in the United States, the European Union and other jurisdictions, our ability to protect our intellectual property and know-how and operate our business without infringing the intellectual property rights or regulatory exclusivity of others, our ability to manage negative consequences from changes in applicable laws and regulations, including tax laws, our ability to successfully remediate the material weaknesses in our internal control over financial reporting and to maintain an effective system of internal control over financial reporting, changes and uncertainty in general market, political and economic conditions, including as a result of inflation and the current conflict between Russia and Ukraine, the Hamas attack against Israel and the ensuing war, and the other factors described under the headings "Cautionary Statement Regarding Forward-Looking Statements" and "Item 3. Key Information--D. Risk Factors" in our Annual Report on Form 20-F and other periodic filings with the Securities and Exchange Commission. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. This presentation includes data for an investigational product not yet approved by regulatory authorities. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.
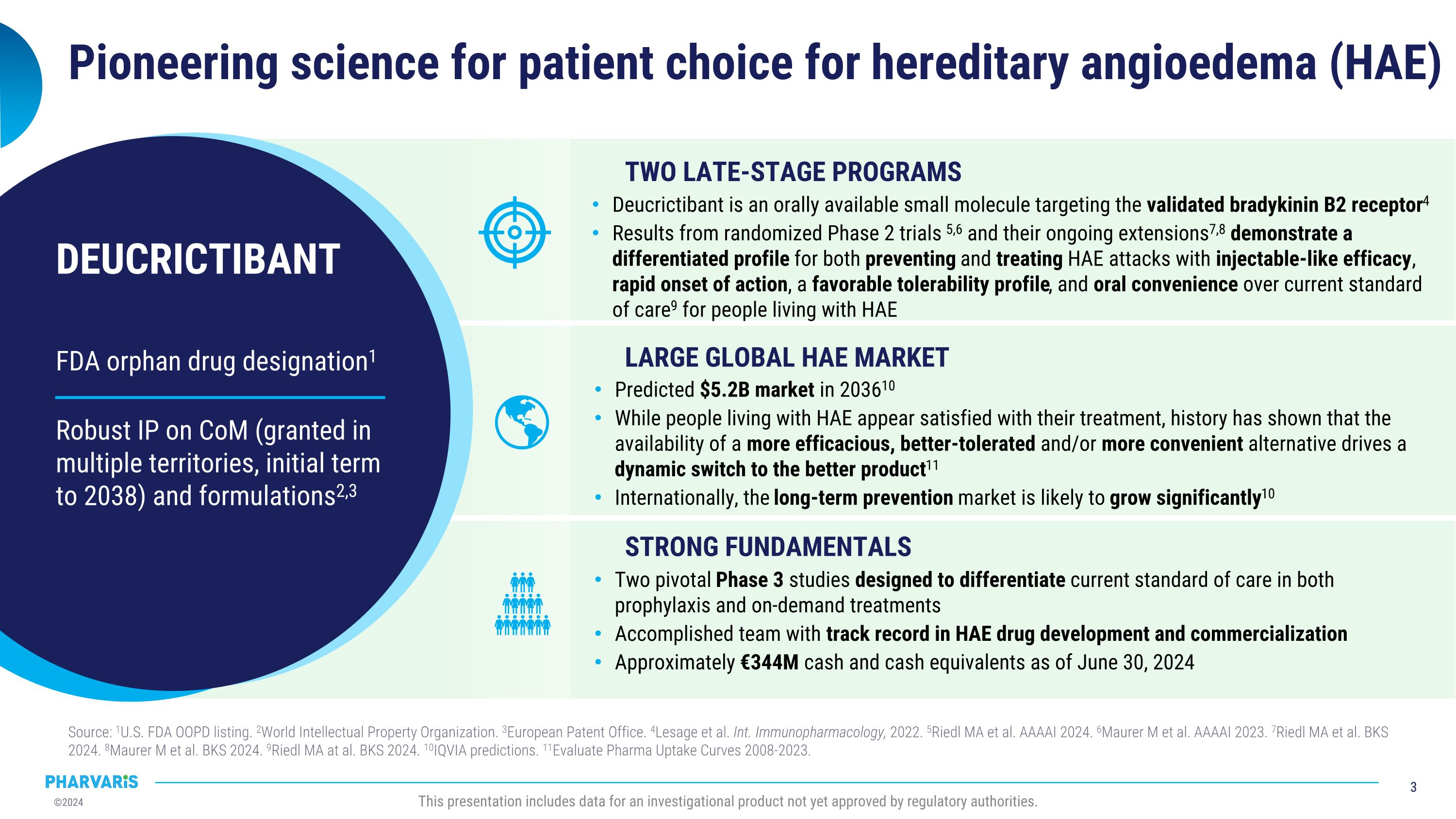
FDA orphan drug designation1 Robust IP on CoM (granted in multiple territories, initial term to 2038) and formulations2,3 DEUCRICTIBANT Pioneering science for patient choice for hereditary angioedema (HAE) Source: 1U.S. FDA OOPD listing. 2World Intellectual Property Organization. 3European Patent Office. 4Lesage et al. Int. Immunopharmacology, 2022. 5Riedl MA et al. AAAAI 2024. 6Maurer M et al. AAAAI 2023. 7Riedl MA et al. BKS 2024. 8Maurer M et al. BKS 2024. 9Riedl MA at al. BKS 2024. 10IQVIA predictions. 11Evaluate Pharma Uptake Curves 2008-2023. This presentation includes data for an investigational product not yet approved by regulatory authorities. TWO LATE-STAGE PROGRAMS LARGE GLOBAL HAE MARKET STRONG FUNDAMENTALS Deucrictibant is an orally available small molecule targeting the validated bradykinin B2 receptor4 Results from randomized Phase 2 trials 5,6 and their ongoing extensions7,8 demonstrate a differentiated profile for both preventing and treating HAE attacks with injectable-like efficacy, rapid onset of action, a favorable tolerability profile, and oral convenience over current standard of care9 for people living with HAE Predicted $5.2B market in 203610 While people living with HAE appear satisfied with their treatment, history has shown that the availability of a more efficacious, better-tolerated and/or more convenient alternative drives a dynamic switch to the better product11 Internationally, the long-term prevention market is likely to grow significantly10 Two pivotal Phase 3 studies designed to differentiate current standard of care in both prophylaxis and on-demand treatments Accomplished team with track record in HAE drug development and commercialization Approximately €344M cash and cash equivalents as of June 30, 2024
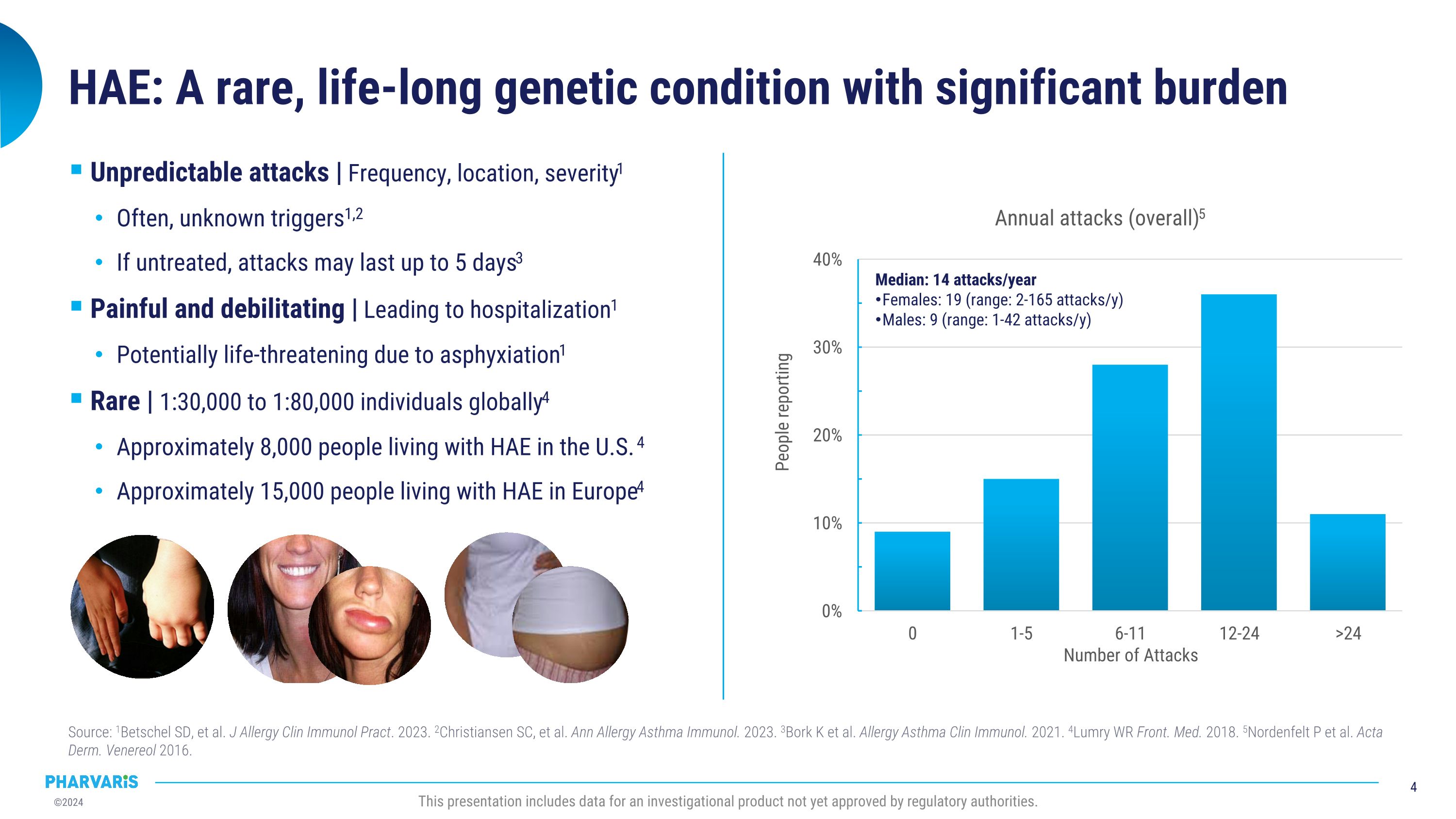
Median: 14 attacks/year Females: 19 (range: 2-165 attacks/y) Males: 9 (range: 1-42 attacks/y) HAE: A rare, life-long genetic condition with significant burden Source: 1Betschel SD, et al. J Allergy Clin Immunol Pract. 2023. 2Christiansen SC, et al. Ann Allergy Asthma Immunol. 2023. 3Bork K et al. Allergy Asthma Clin Immunol. 2021. 4Lumry WR Front. Med. 2018. 5Nordenfelt P et al. Acta Derm. Venereol 2016. This presentation includes data for an investigational product not yet approved by regulatory authorities. Unpredictable attacks | Frequency, location, severity1 Often, unknown triggers1,2 If untreated, attacks may last up to 5 days3 Painful and debilitating | Leading to hospitalization1 Potentially life-threatening due to asphyxiation1 Rare | 1:30,000 to 1:80,000 individuals globally4 Approximately 8,000 people living with HAE in the U.S. 4 Approximately 15,000 people living with HAE in Europe4
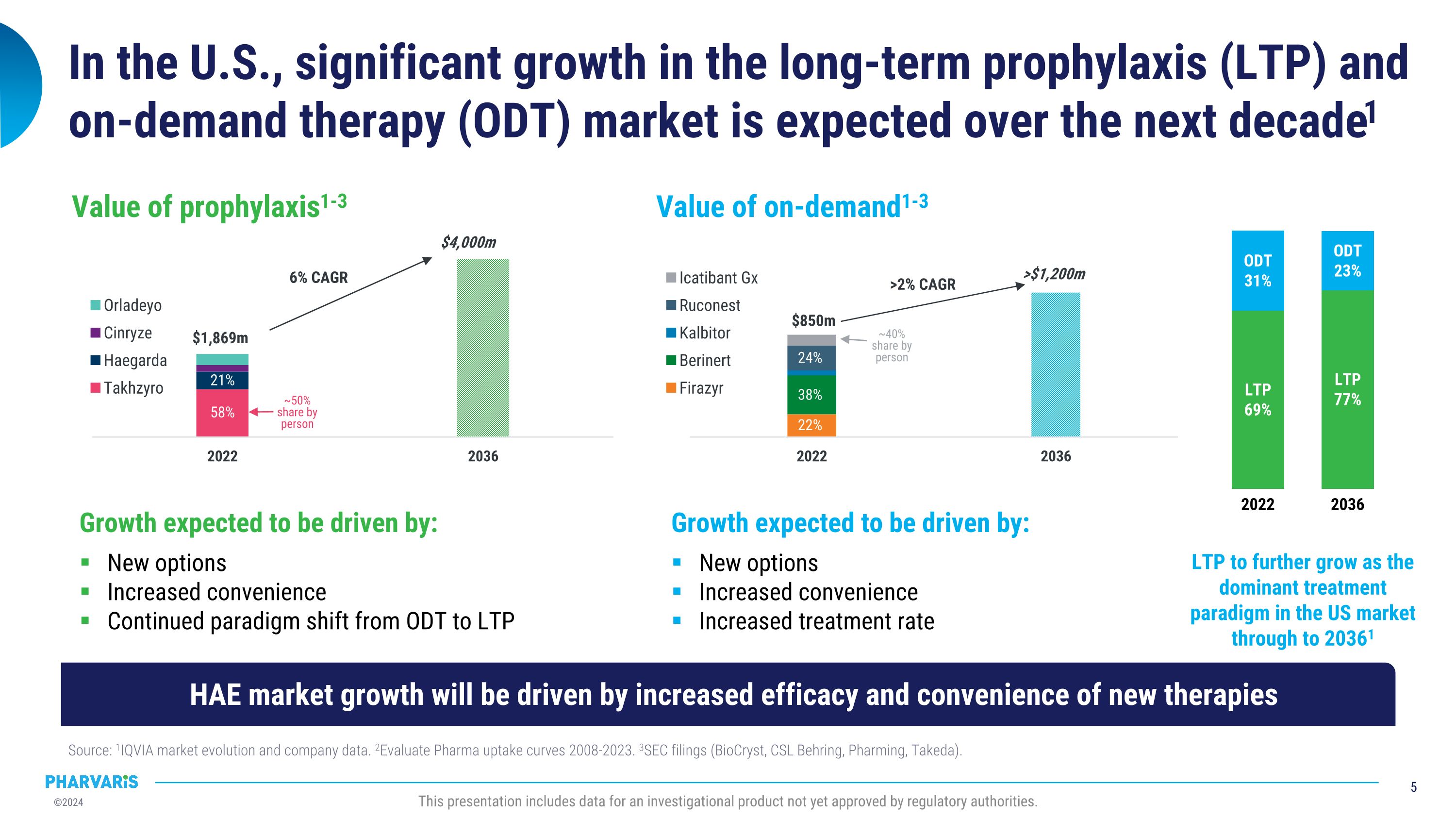
In the U.S., significant growth in the long-term prophylaxis (LTP) and �on-demand therapy (ODT) market is expected over the next decade1 Source: 1IQVIA market evolution and company data. 2Evaluate Pharma uptake curves 2008-2023. 3SEC filings (BioCryst, CSL Behring, Pharming, Takeda). This presentation includes data for an investigational product not yet approved by regulatory authorities. Growth expected to be driven by: New options Increased convenience Increased treatment rate Value of on-demand1-3 $850m >$1,200m >2% CAGR 22% 38% 24% ~40% share by person Growth expected to be driven by: New options Increased convenience Continued paradigm shift from ODT to LTP Value of prophylaxis1-3 $1,869m $4,000m 6% CAGR 58% 21% ~50% share by person LTP to further grow as the dominant treatment paradigm in the US market through to 20361 2022 LTP 69% ODT 31% 2036 LTP 77% ODT 23% HAE market growth will be driven by increased efficacy and convenience of new therapies
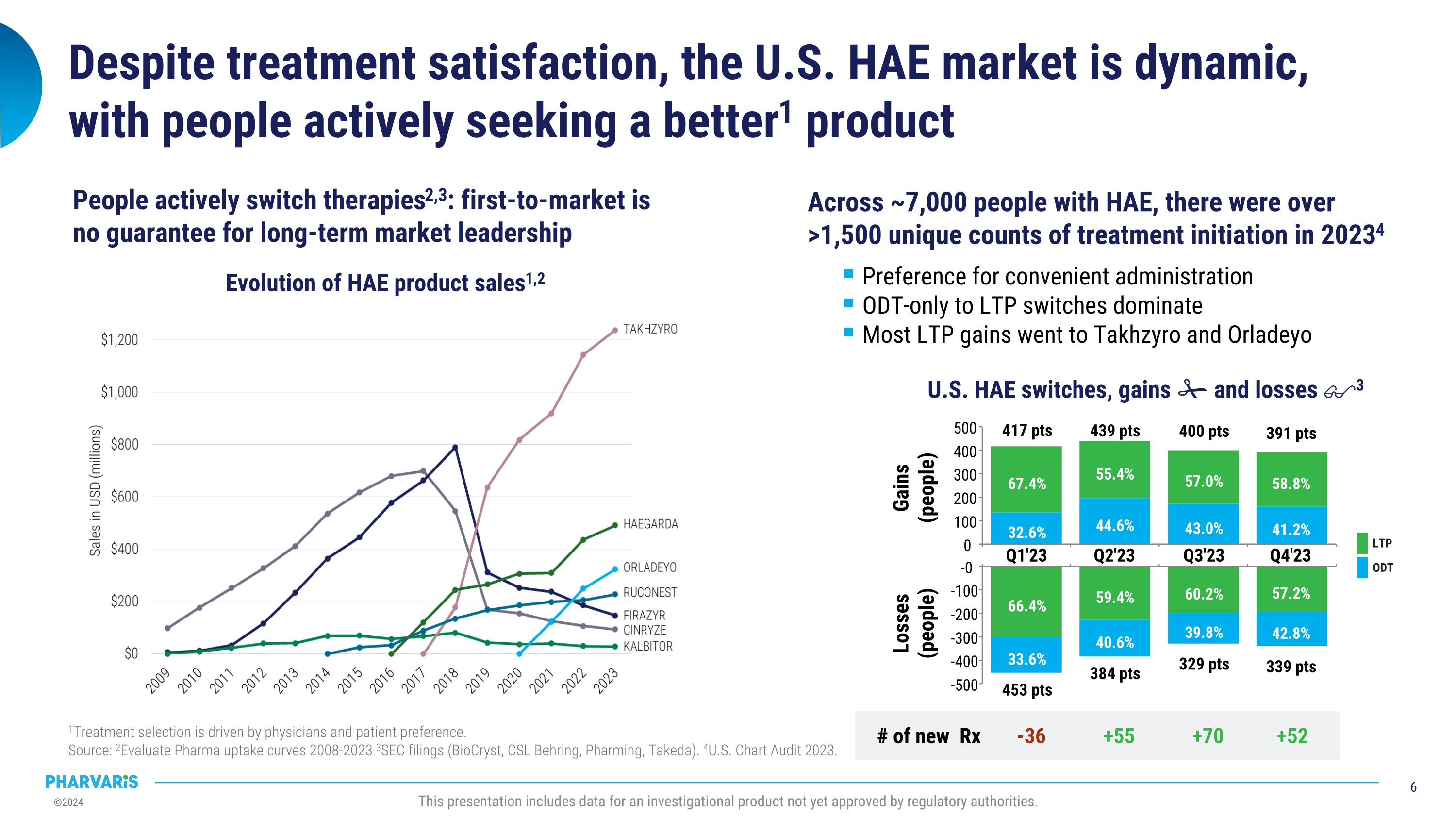
453 pts 384 pts 339 pts 329 pts Despite treatment satisfaction, the U.S. HAE market is dynamic, with people actively seeking a better1 product 1Treatment selection is driven by physicians and patient preference. �Source: 2Evaluate Pharma uptake curves 2008-2023 3SEC filings (BioCryst, CSL Behring, Pharming, Takeda). 4U.S. Chart Audit 2023. This presentation includes data for an investigational product not yet approved by regulatory authorities. Across ~7,000 people with HAE, there were over >1,500 unique counts of treatment initiation in 20234 Preference for convenient administration ODT-only to LTP switches dominate Most LTP gains went to Takhzyro and Orladeyo U.S. HAE switches, gains and losses 3 -36 +55 +70 +52 # of new Rx 66.4% 59.4% 60.2% 57.2% 33.6% 40.6% 39.8% 42.8% Q1'23 Q2'23 Q3'23 Q4'23 -0 -100 -200 -300 -400 -500 Losses�(people) 32.6% 44.6% 43.0% 41.2% 67.4% 55.4% 57.0% 58.8% 417 pts 439 pts 400 pts 391 pts Q1'23 Q2'23 Q3'23 Q4'23 0 100 200 300 400 500 Gains�(people) ODT LTP Evolution of HAE product sales1,2 People actively switch therapies2,3: first-to-market is no guarantee for long-term market leadership
People living with HAE are seeking a life not defined by their condition nor burdened by its management1 Source: 1Lumry WR et al. Allergy Asthma Proc. 2020. 2Geba et al, J Drug Access.2021. 3U.S. Chart Audit 2023 This presentation includes data for an investigational product not yet approved by regulatory authorities. People living with HAE actively switch between products3,�seeking improvement in efficacy, safety/tolerability, and convenience Efficacy is a prime driver… but safety and tolerability cause exploration of alternatives… …while convenience is a key driver for overall preference2
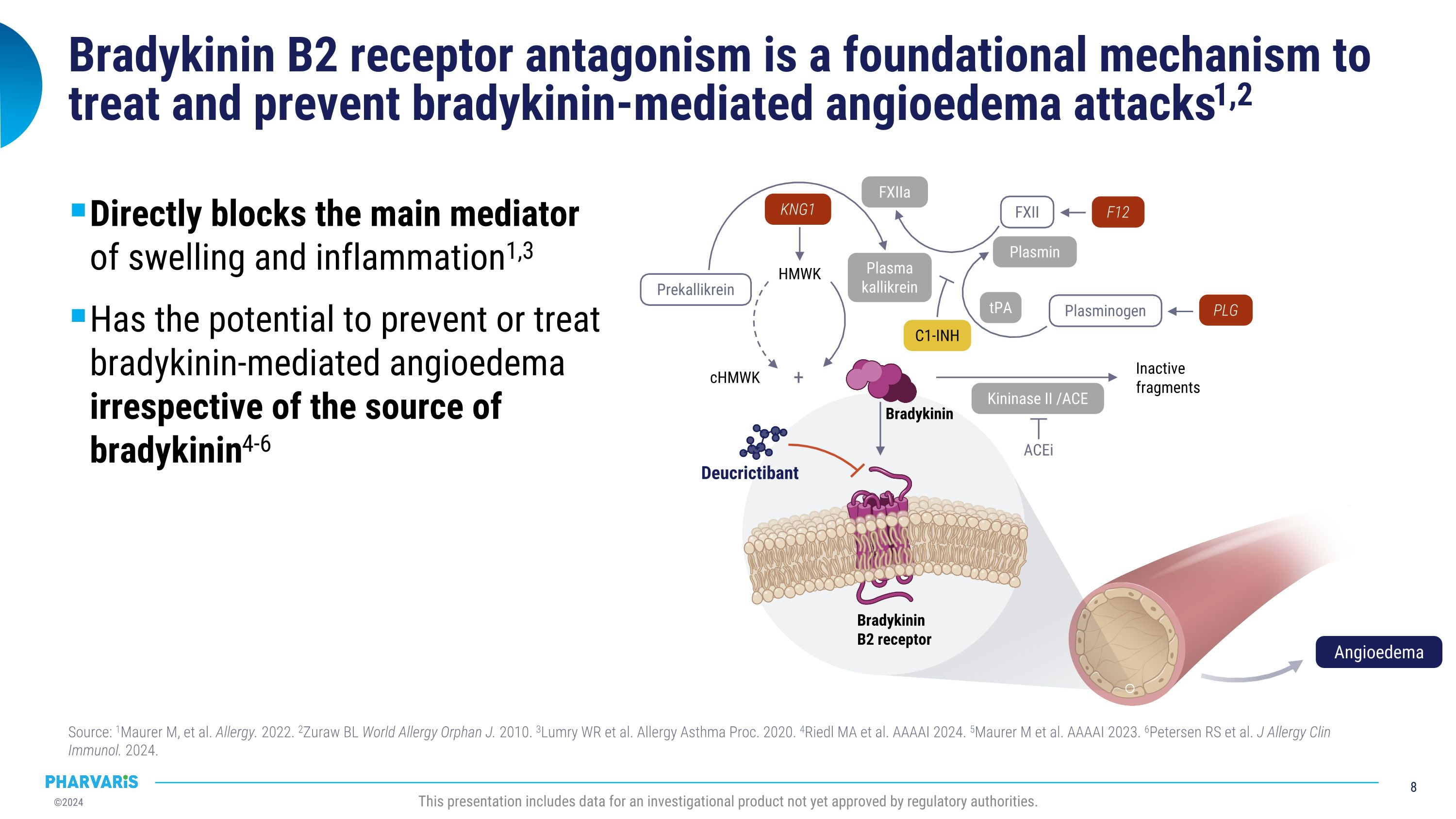
Bradykinin B2 receptor antagonism is a foundational mechanism to treat and prevent bradykinin-mediated angioedema attacks1,2 Directly blocks the main mediator�of swelling and inflammation1,3 Has the potential to prevent or treat�bradykinin-mediated angioedema�irrespective of the source of�bradykinin4-6 Source: 1Maurer M, et al. Allergy. 2022. 2Zuraw BL World Allergy Orphan J. 2010. 3Lumry WR et al. Allergy Asthma Proc. 2020. 4Riedl MA et al. AAAAI 2024. 5Maurer M et al. AAAAI 2023. 6Petersen RS et al. J Allergy Clin Immunol. 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. cHMWK Inactive fragments HMWK ACEi + Prekallikrein FXII Plasminogen Plasmin FXIIa tPA Kininase II /ACE Plasma kallikrein KNG1 F12 PLG C1-INH Bradykinin B2 receptor Deucrictibant Bradykinin Angioedema
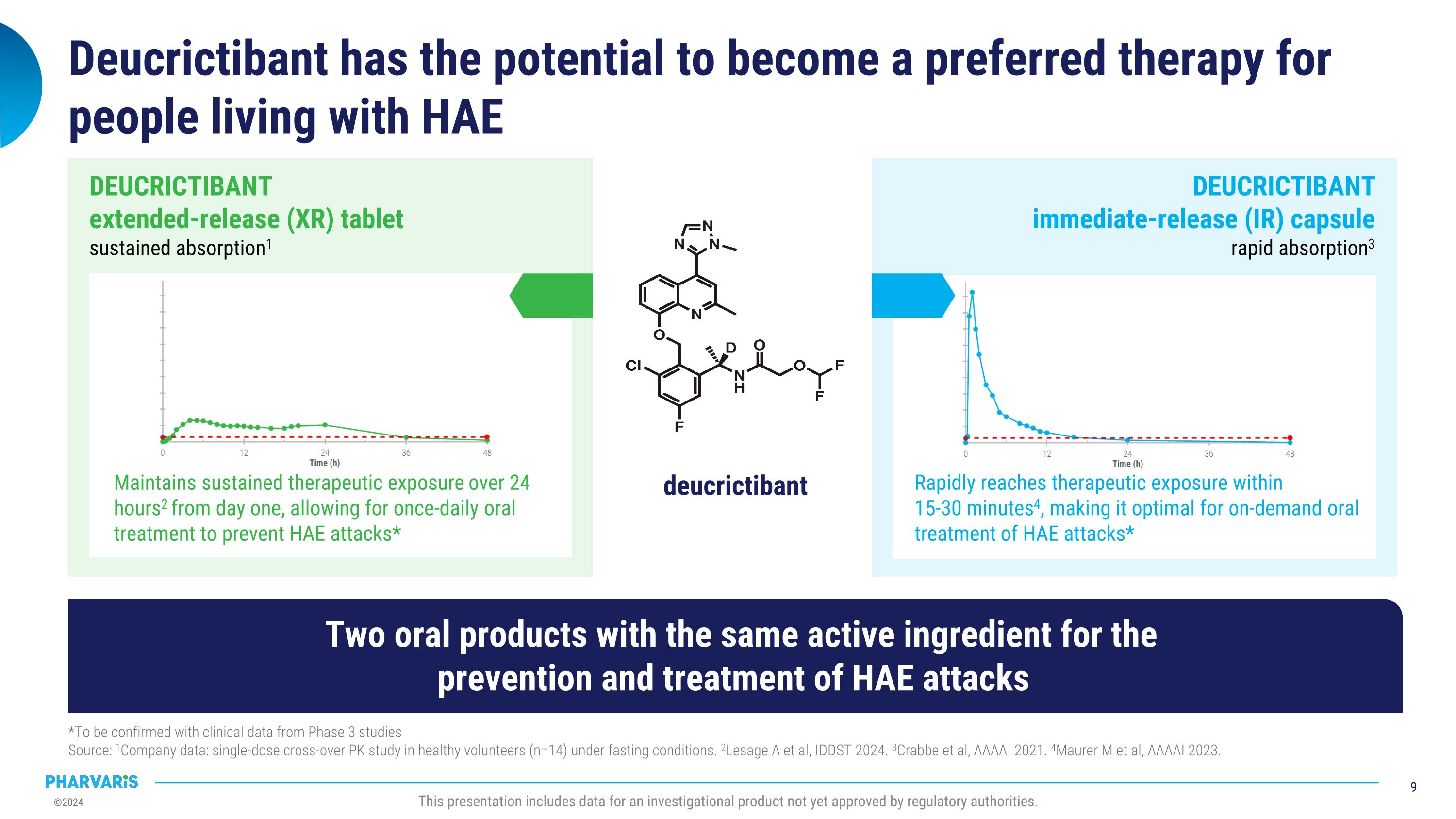
Deucrictibant has the potential to become a preferred therapy for people living with HAE *To be confirmed with clinical data from Phase 3 studies�Source: 1Company data: single-dose cross-over PK study in healthy volunteers (n=14) under fasting conditions. 2Lesage A et al, IDDST 2024. 3Crabbe et al, AAAAI 2021. 4Maurer M et al, AAAAI 2023. This presentation includes data for an investigational product not yet approved by regulatory authorities. Rapidly reaches therapeutic exposure within �15-30 minutes4, making it optimal for on-demand oral treatment of HAE attacks* DEUCRICTIBANT�immediate-release (IR) capsule�rapid absorption3 deucrictibant Maintains sustained therapeutic exposure over 24 hours2 from day one, allowing for once-daily oral treatment to prevent HAE attacks* DEUCRICTIBANT�extended-release (XR) tablet�sustained absorption1 Two oral products with the same active ingredient for the �prevention and treatment of HAE attacks
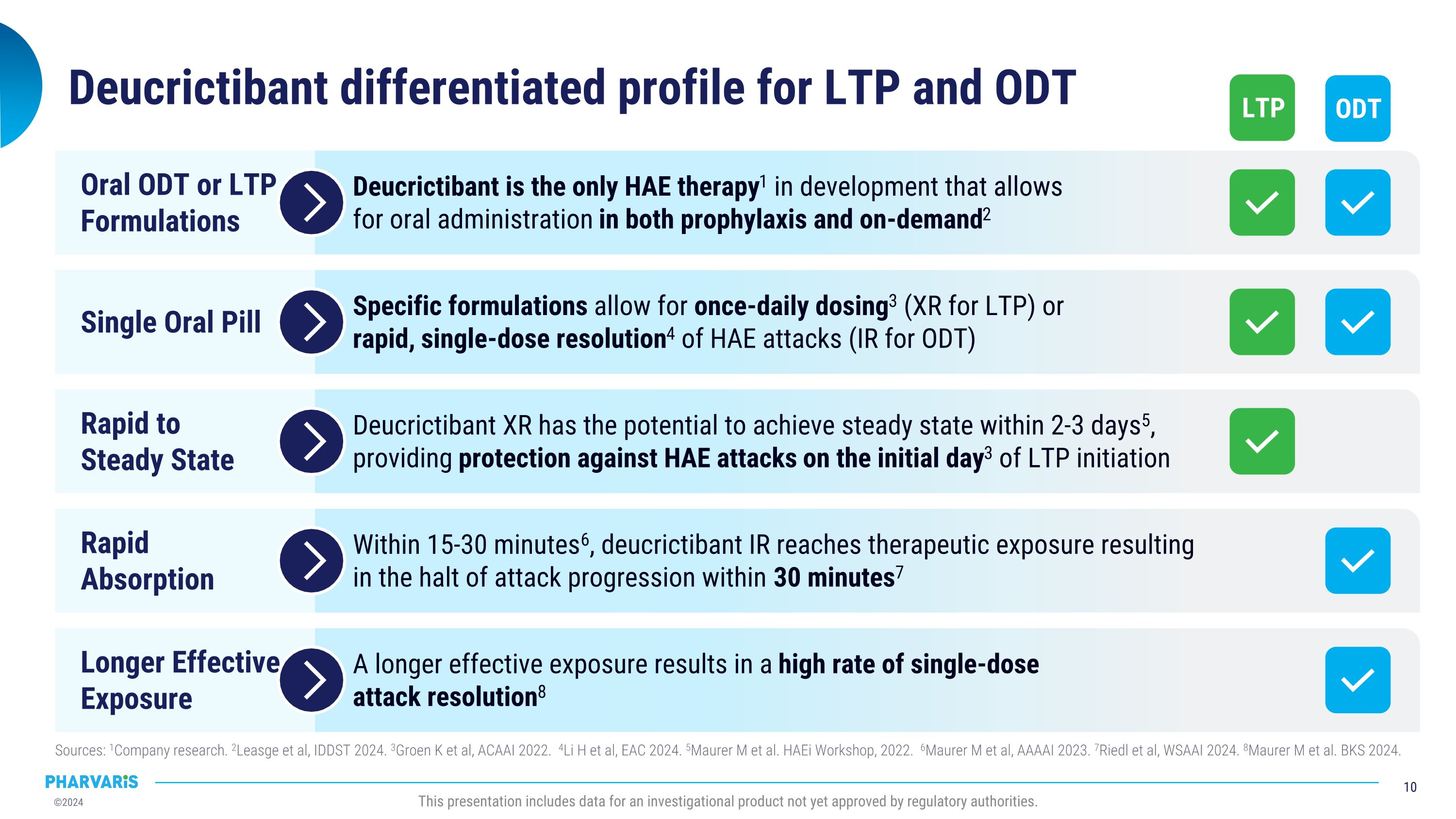
Deucrictibant differentiated profile for LTP and ODT Sources: 1Company research. 2Leasge et al, IDDST 2024. 3Groen K et al, ACAAI 2022. 4Li H et al, EAC 2024. 5Maurer M et al. HAEi Workshop, 2022. 6Maurer M et al, AAAAI 2023. 7Riedl et al, WSAAI 2024. 8Maurer M et al. BKS 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. Rapid�Absorption Within 15-30 minutes6, deucrictibant IR reaches therapeutic exposure resulting �in the halt of attack progression within 30 minutes7 Longer Effective Exposure A longer effective exposure results in a high rate of single-dose�attack resolution8 Single Oral Pill Specific formulations allow for once-daily dosing3 (XR for LTP) or�rapid, single-dose resolution4 of HAE attacks (IR for ODT) Oral ODT or LTP�Formulations Deucrictibant is the only HAE therapy1 in development that allows�for oral administration in both prophylaxis and on-demand2 Rapid to�Steady State Deucrictibant XR has the potential to achieve steady state within 2-3 days5, providing protection against HAE attacks on the initial day3 of LTP initiation LTP ODT
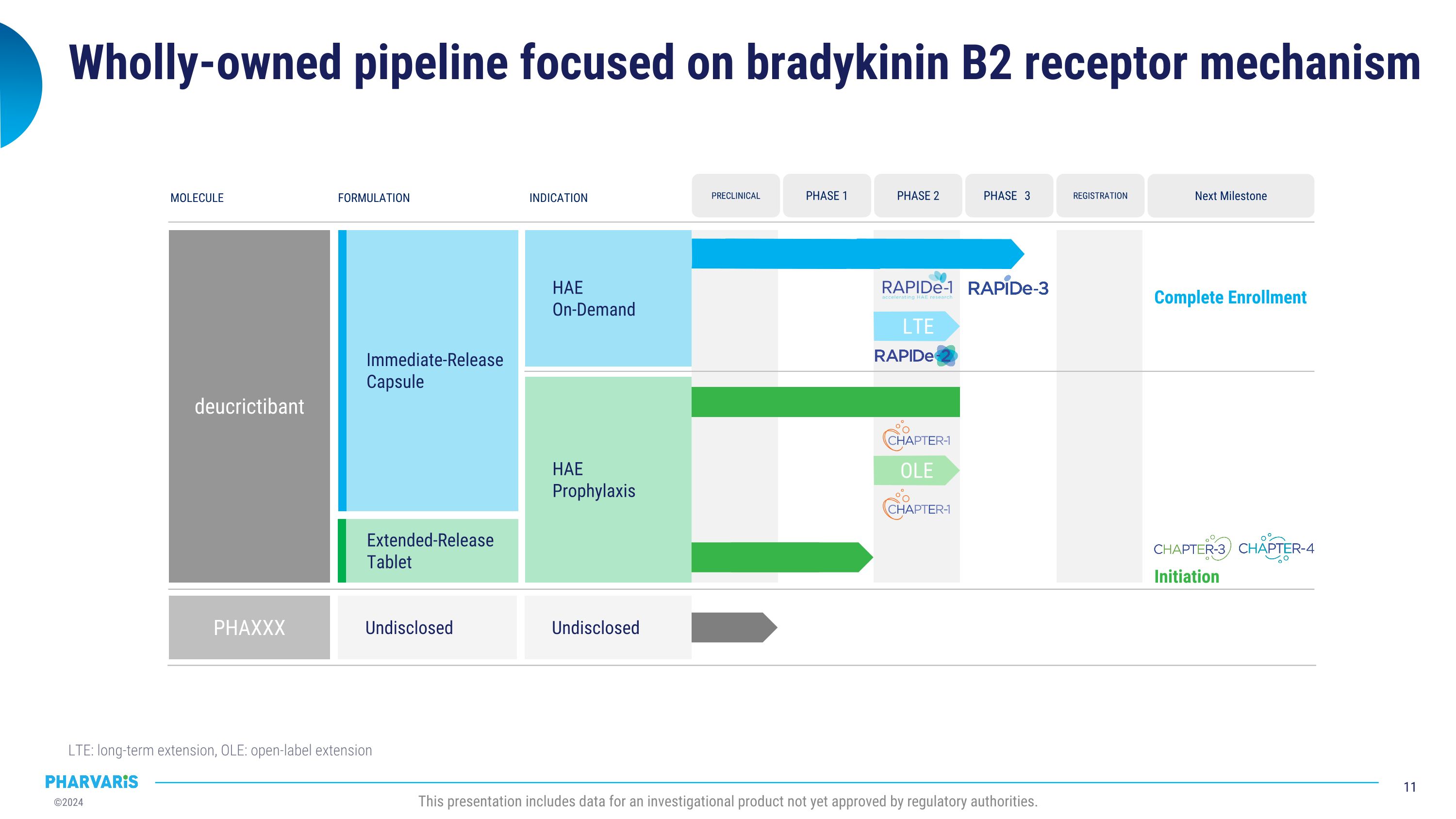
Wholly-owned pipeline focused on bradykinin B2 receptor mechanism LTE: long-term extension, OLE: open-label extension This presentation includes data for an investigational product not yet approved by regulatory authorities. MOLECULE FORMULATION INDICATION deucrictibant Undisclosed PHAXXX HAE On-Demand HAE Prophylaxis Next Milestone Complete Enrollment REGISTRATION PHASE 3 PRECLINICAL PHASE 1 PHASE 2 Undisclosed Immediate-Release Capsule Extended-Release Tablet OLE Initiation LTE
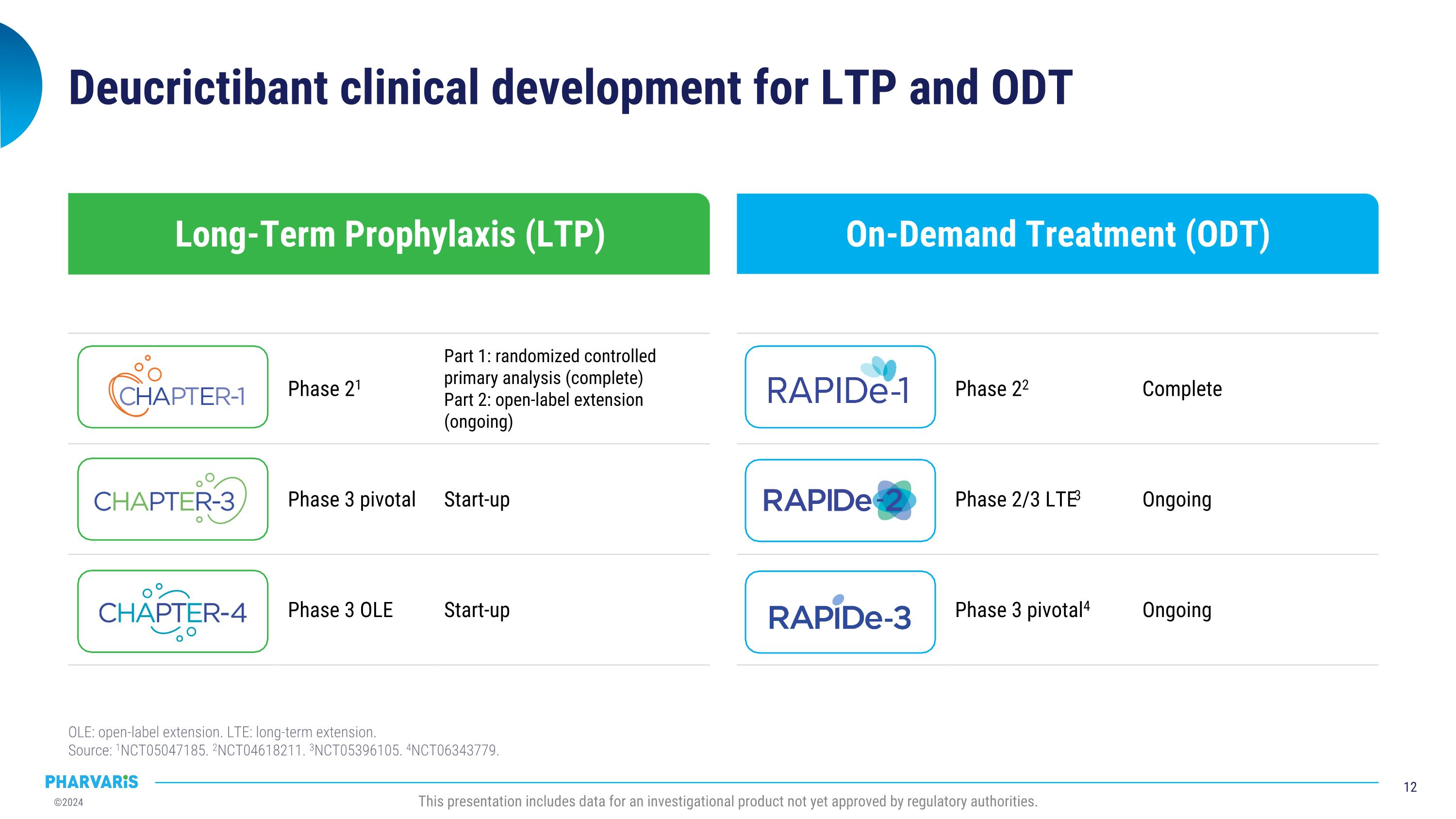
On-Demand Treatment (ODT) Long-Term Prophylaxis (LTP) Deucrictibant clinical development for LTP and ODT OLE: open-label extension. LTE: long-term extension.�Source: 1NCT05047185. 2NCT04618211. 3NCT05396105. 4NCT06343779. Phase 22 Complete Phase 2/3 LTE3 Ongoing Phase 3 pivotal4 Ongoing Phase 21 Part 1: randomized controlled primary analysis (complete) Part 2: open-label extension (ongoing) Phase 3 pivotal Start-up Phase 3 OLE Start-up This presentation includes data for an investigational product not yet approved by regulatory authorities.
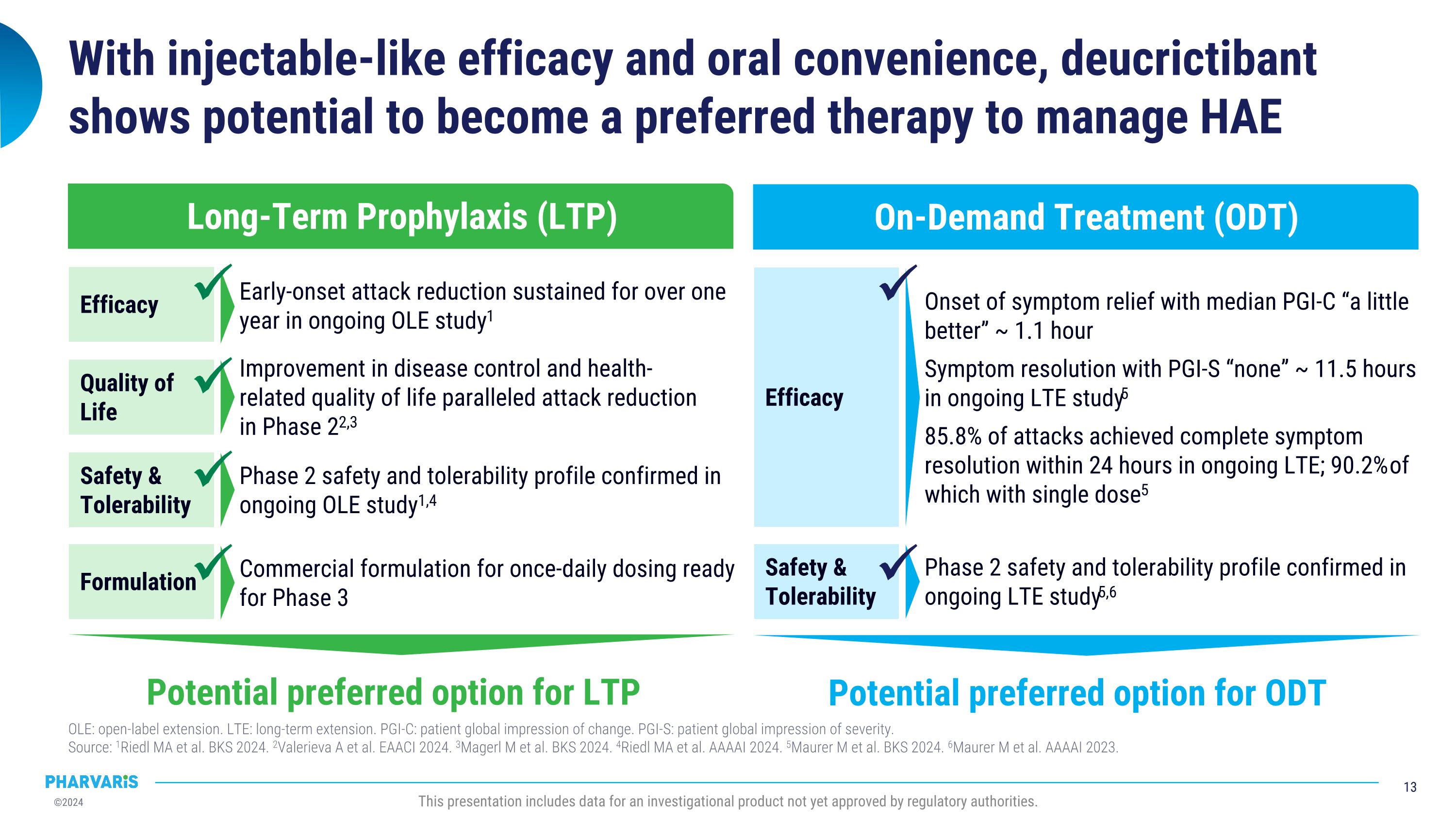
Efficacy With injectable-like efficacy and oral convenience, deucrictibant shows potential to become a preferred therapy to manage HAE OLE: open-label extension. LTE: long-term extension. PGI-C: patient global impression of change. PGI-S: patient global impression of severity. �Source: 1Riedl MA et al. BKS 2024. 2Valerieva A et al. EAACI 2024. 3Magerl M et al. BKS 2024. 4Riedl MA et al. AAAAI 2024. 5Maurer M et al. BKS 2024. 6Maurer M et al. AAAAI 2023. This presentation includes data for an investigational product not yet approved by regulatory authorities. Long-Term Prophylaxis (LTP) Potential preferred option for LTP Safety & Tolerability Quality of Life Formulation Efficacy Early-onset attack reduction sustained for over one year in ongoing OLE study1 Improvement in disease control and health-related quality of life paralleled attack reduction in Phase 22,3 Phase 2 safety and tolerability profile confirmed in ongoing OLE study1,4 Commercial formulation for once-daily dosing ready for Phase 3 On-Demand Treatment (ODT) Potential preferred option for ODT Safety & Tolerability Onset of symptom relief with median PGI-C “a little better” ~ 1.1 hour Symptom resolution with PGI-S “none” ~ 11.5 hours in ongoing LTE study5 85.8% of attacks achieved complete symptom resolution within 24 hours in ongoing LTE; 90.2% of which with single dose5 Phase 2 safety and tolerability profile confirmed in ongoing LTE study5,6
Long-Term Prophylaxis Deucrictibant extended-release tablets
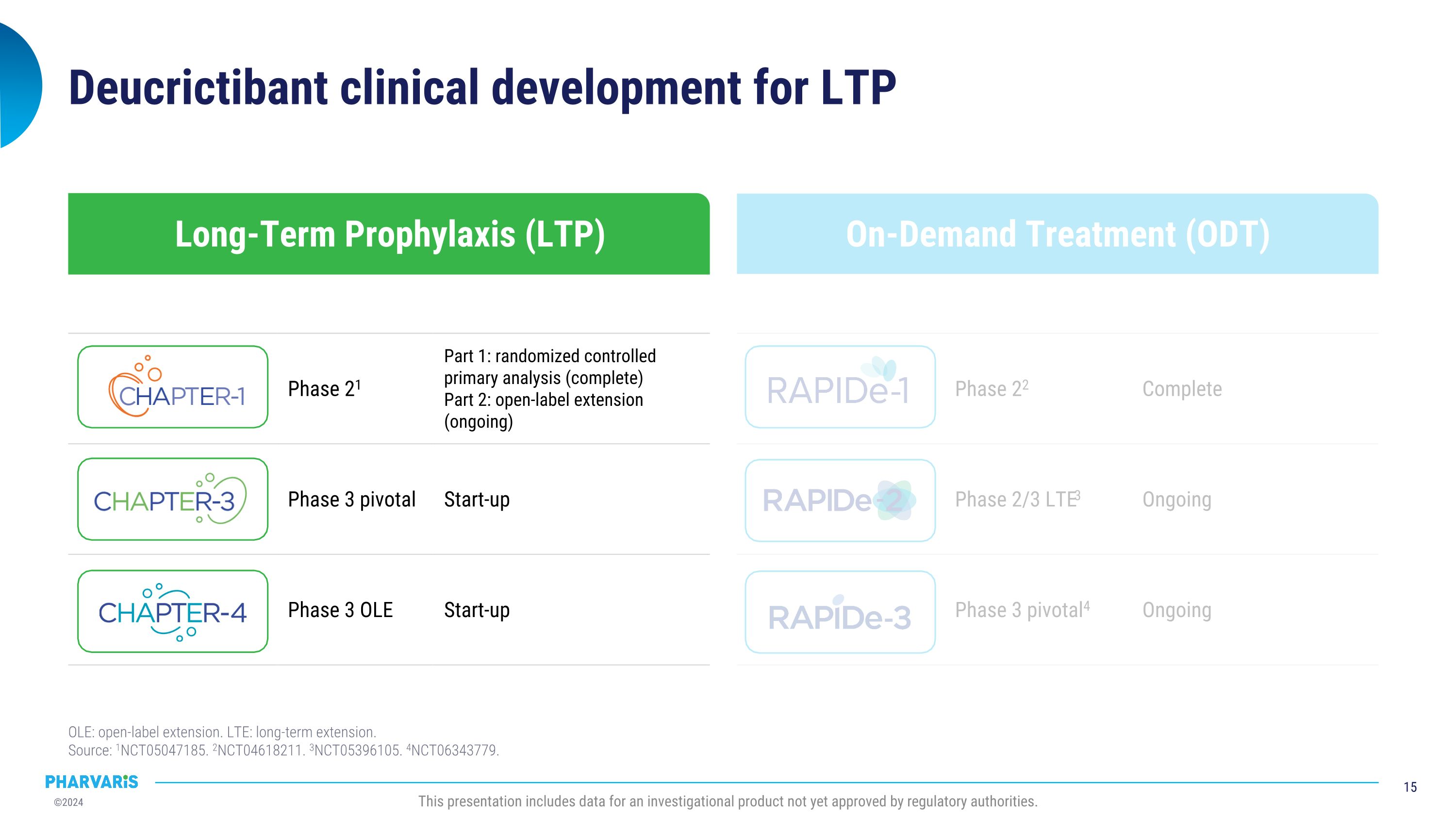
On-Demand Treatment (ODT) Long-Term Prophylaxis (LTP) Deucrictibant clinical development for LTP OLE: open-label extension. LTE: long-term extension.�Source: 1NCT05047185. 2NCT04618211. 3NCT05396105. 4NCT06343779. Phase 22 Complete Phase 2/3 LTE3 Ongoing Phase 3 pivotal4 Ongoing Phase 21 Part 1: randomized controlled primary analysis (complete) Part 2: open-label extension (ongoing) Phase 3 pivotal Start-up Phase 3 OLE Start-up This presentation includes data for an investigational product not yet approved by regulatory authorities.
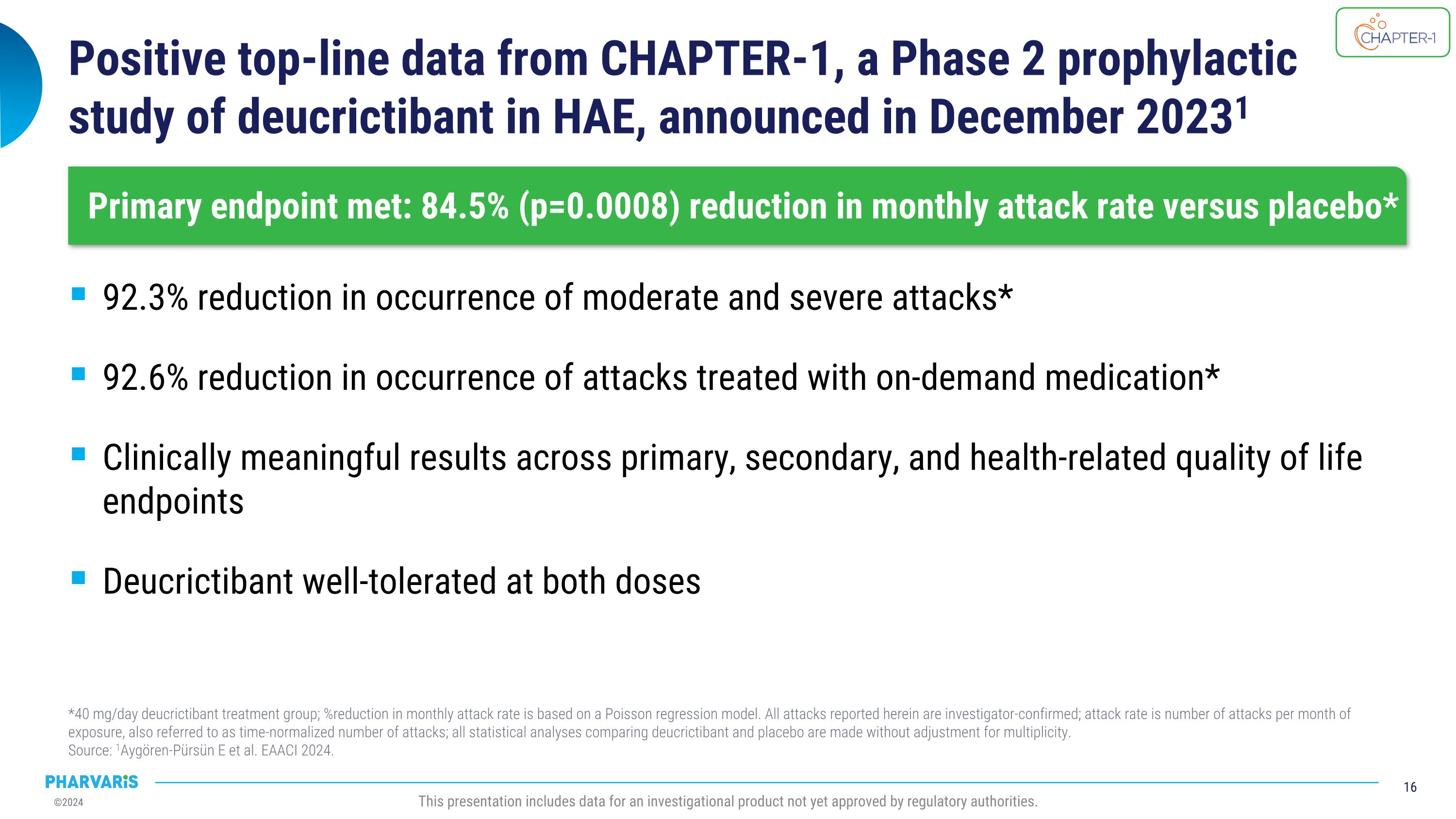
Positive top-line data from CHAPTER-1, a Phase 2 prophylactic �study of deucrictibant in HAE, announced in December 20231 92.3% reduction in occurrence of moderate and severe attacks* 92.6% reduction in occurrence of attacks treated with on-demand medication* Clinically meaningful results across primary, secondary, and health-related quality of life endpoints Deucrictibant well-tolerated at both doses *40 mg/day deucrictibant treatment group; %reduction in monthly attack rate is based on a Poisson regression model. All attacks reported herein are investigator-confirmed; attack rate is number of attacks per month of exposure, also referred to as time-normalized number of attacks; all statistical analyses comparing deucrictibant and placebo are made without adjustment for multiplicity.�Source: 1Aygören-Pürsün E et al. EAACI 2024. Primary endpoint met: 84.5% (p=0.0008) reduction in monthly attack rate versus placebo* This presentation includes data for an investigational product not yet approved by regulatory authorities.
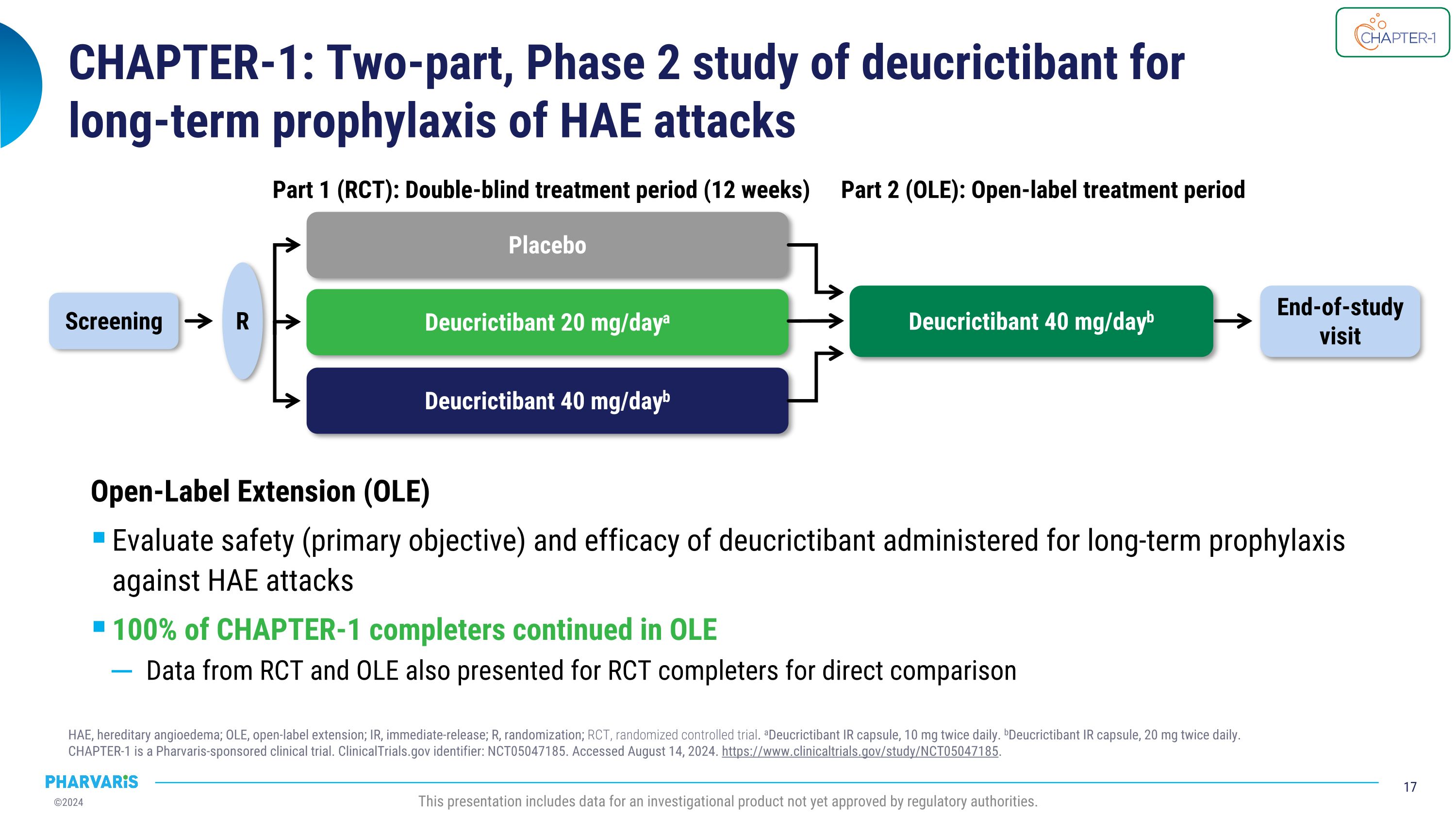
CHAPTER-1: Two-part, Phase 2 study of deucrictibant for�long-term prophylaxis of HAE attacks HAE, hereditary angioedema; OLE, open-label extension; IR, immediate-release; R, randomization; RCT, randomized controlled trial. aDeucrictibant IR capsule, 10 mg twice daily. bDeucrictibant IR capsule, 20 mg twice daily.�CHAPTER-1 is a Pharvaris-sponsored clinical trial. ClinicalTrials.gov identifier: NCT05047185. Accessed August 14, 2024. https://www.clinicaltrials.gov/study/NCT05047185. This presentation includes data for an investigational product not yet approved by regulatory authorities. Placebo Deucrictibant 20 mg/daya Deucrictibant 40 mg/dayb Part 1 (RCT): Double-blind treatment period (12 weeks) Screening R End-of-study visit Deucrictibant 40 mg/dayb Part 2 (OLE): Open-label treatment period Open-Label Extension (OLE) Evaluate safety (primary objective) and efficacy of deucrictibant administered for long-term prophylaxis against HAE attacks 100% of CHAPTER-1 completers continued in OLE Data from RCT and OLE also presented for RCT completers for direct comparison
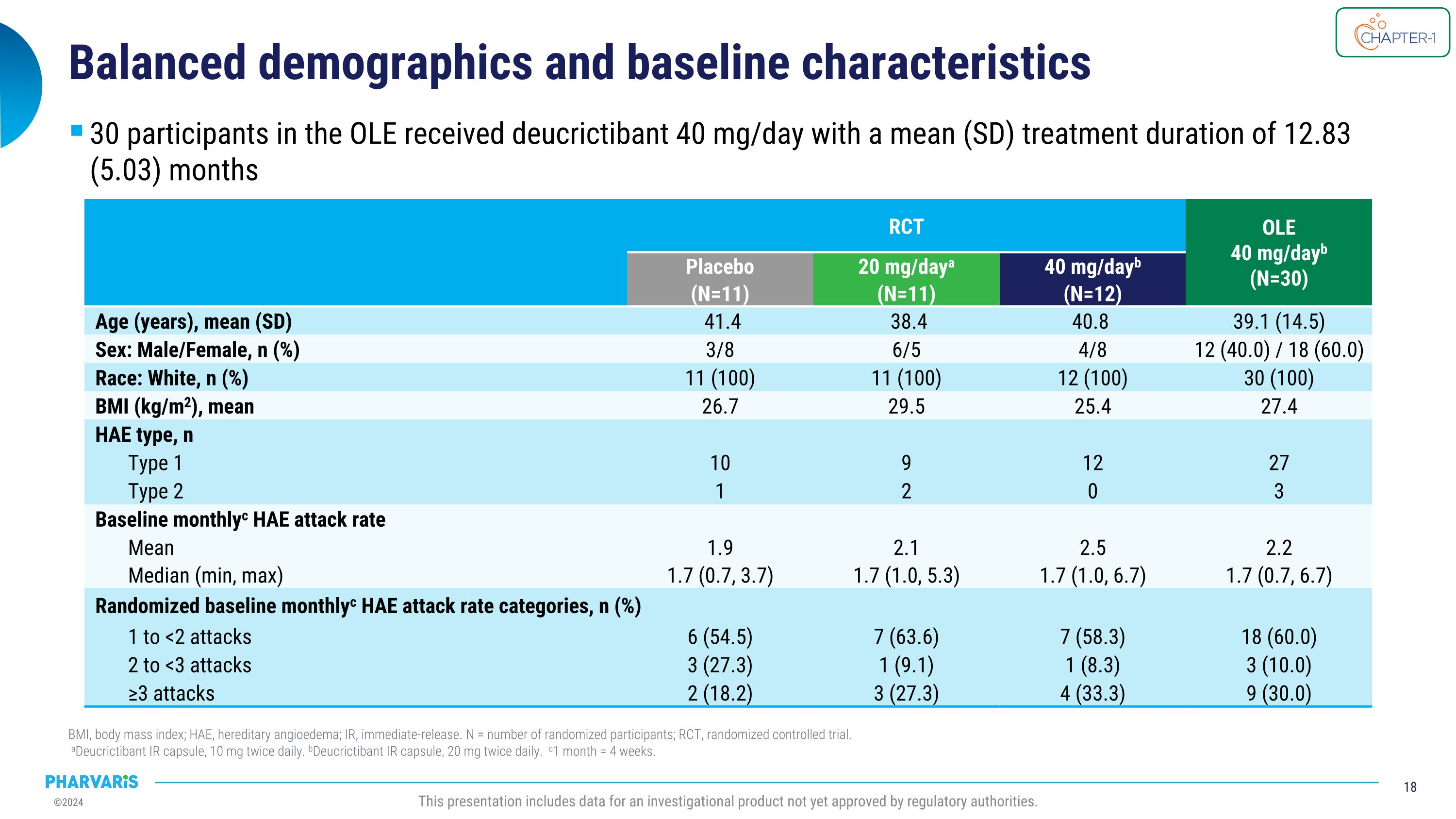
Balanced demographics and baseline characteristics BMI, body mass index; HAE, hereditary angioedema; IR, immediate-release. N = number of randomized participants; RCT, randomized controlled trial.� aDeucrictibant IR capsule, 10 mg twice daily. bDeucrictibant IR capsule, 20 mg twice daily. c1 month = 4 weeks. This presentation includes data for an investigational product not yet approved by regulatory authorities. RCT OLE 40 mg/dayb�(N=30) Placebo (N=11) 20 mg/daya (N=11) 40 mg/dayb (N=12) Age (years), mean (SD) 41.4 38.4 40.8 39.1 (14.5) Sex: Male/Female, n (%) 3/8 6/5 4/8 12 (40.0) / 18 (60.0) Race: White, n (%) 11 (100) 11 (100) 12 (100) 30 (100) BMI (kg/m2), mean 26.7 29.5 25.4 27.4 HAE type, n Type 1 10 9 12 27 Type 2 1 2 0 3 Baseline monthlyc HAE attack rate Mean 1.9 2.1 2.5 2.2 Median (min, max) 1.7 (0.7, 3.7) 1.7 (1.0, 5.3) 1.7 (1.0, 6.7) 1.7 (0.7, 6.7) Randomized baseline monthlyc HAE attack rate categories, n (%) 1 to <2 attacks 6 (54.5) 7 (63.6) 7 (58.3) 18 (60.0) 2 to <3 attacks 3 (27.3) 1 (9.1) 1 (8.3) 3 (10.0) ≥3 attacks 2 (18.2) 3 (27.3) 4 (33.3) 9 (30.0) 30 participants in the OLE received deucrictibant 40 mg/day with a mean (SD) treatment duration of 12.83 (5.03) months
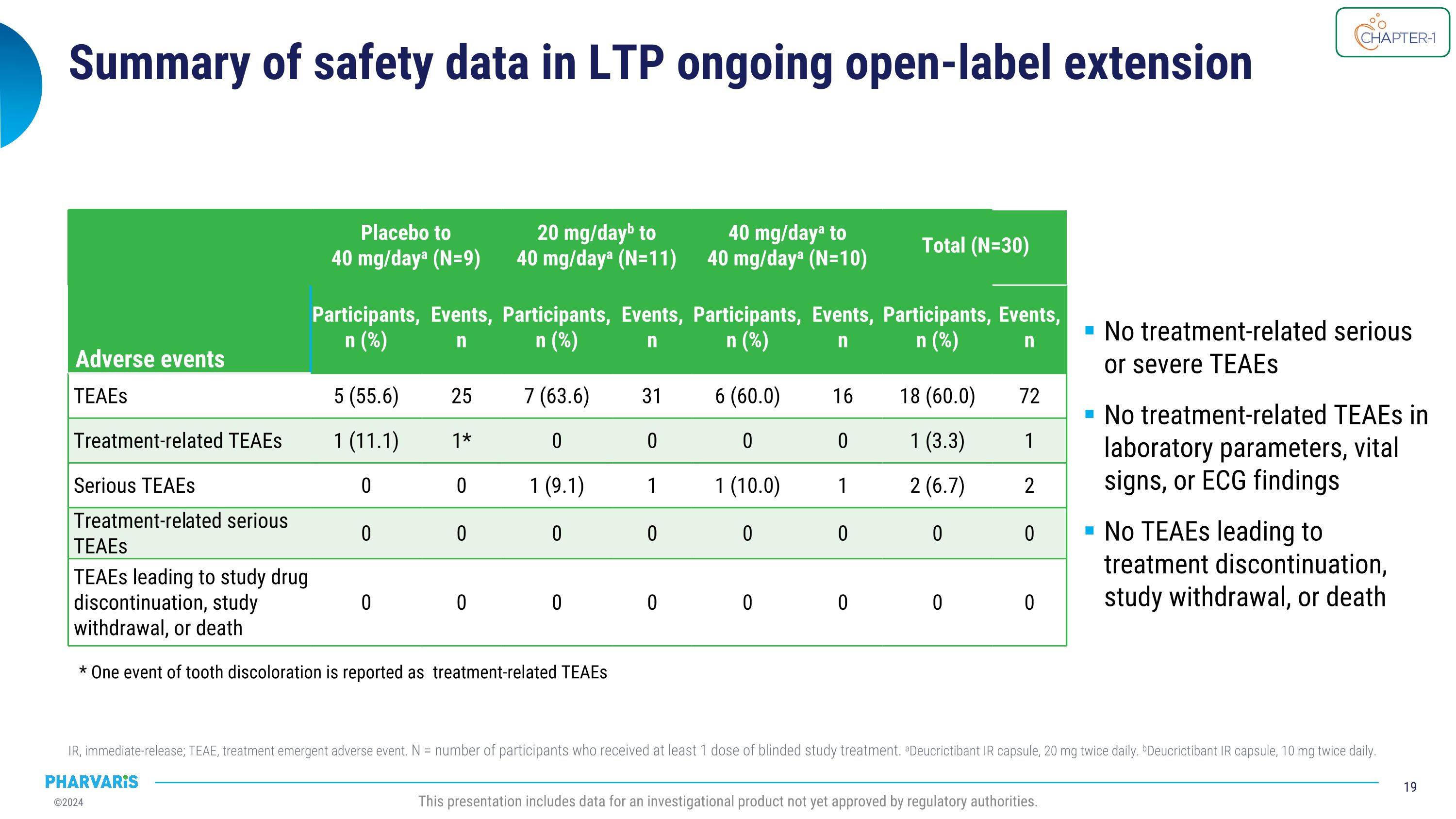
Summary of safety data in LTP ongoing open-label extension IR, immediate-release; TEAE, treatment emergent adverse event. N = number of participants who received at least 1 dose of blinded study treatment. aDeucrictibant IR capsule, 20 mg twice daily. bDeucrictibant IR capsule, 10 mg twice daily. This presentation includes data for an investigational product not yet approved by regulatory authorities. Adverse events Placebo to�40 mg/daya (N=9) 20 mg/dayb to 40 mg/daya (N=11) 40 mg/daya to 40 mg/daya (N=10) Total (N=30) Events Participants,�n (%) Events, �n Participants,�n (%) Events, n Participants,�n (%) Events,�n Participants,�n (%) Events,�n TEAEs 5 (55.6) 25 7 (63.6) 31 6 (60.0) 16 18 (60.0) 72 Treatment-related TEAEs 1 (11.1) 1* 0 0 0 0 1 (3.3) 1 Serious TEAEs 0 0 1 (9.1) 1 1 (10.0) 1 2 (6.7) 2 Treatment-related serious TEAEs 0 0 0 0 0 0 0 0 TEAEs leading to study drug discontinuation, study withdrawal, or death 0 0 0 0 0 0 0 0 No treatment-related serious or severe TEAEs No treatment-related TEAEs in laboratory parameters, vital signs, or ECG findings No TEAEs leading to treatment discontinuation, study withdrawal, or death * One event of tooth discoloration is reported as treatment-related TEAEs
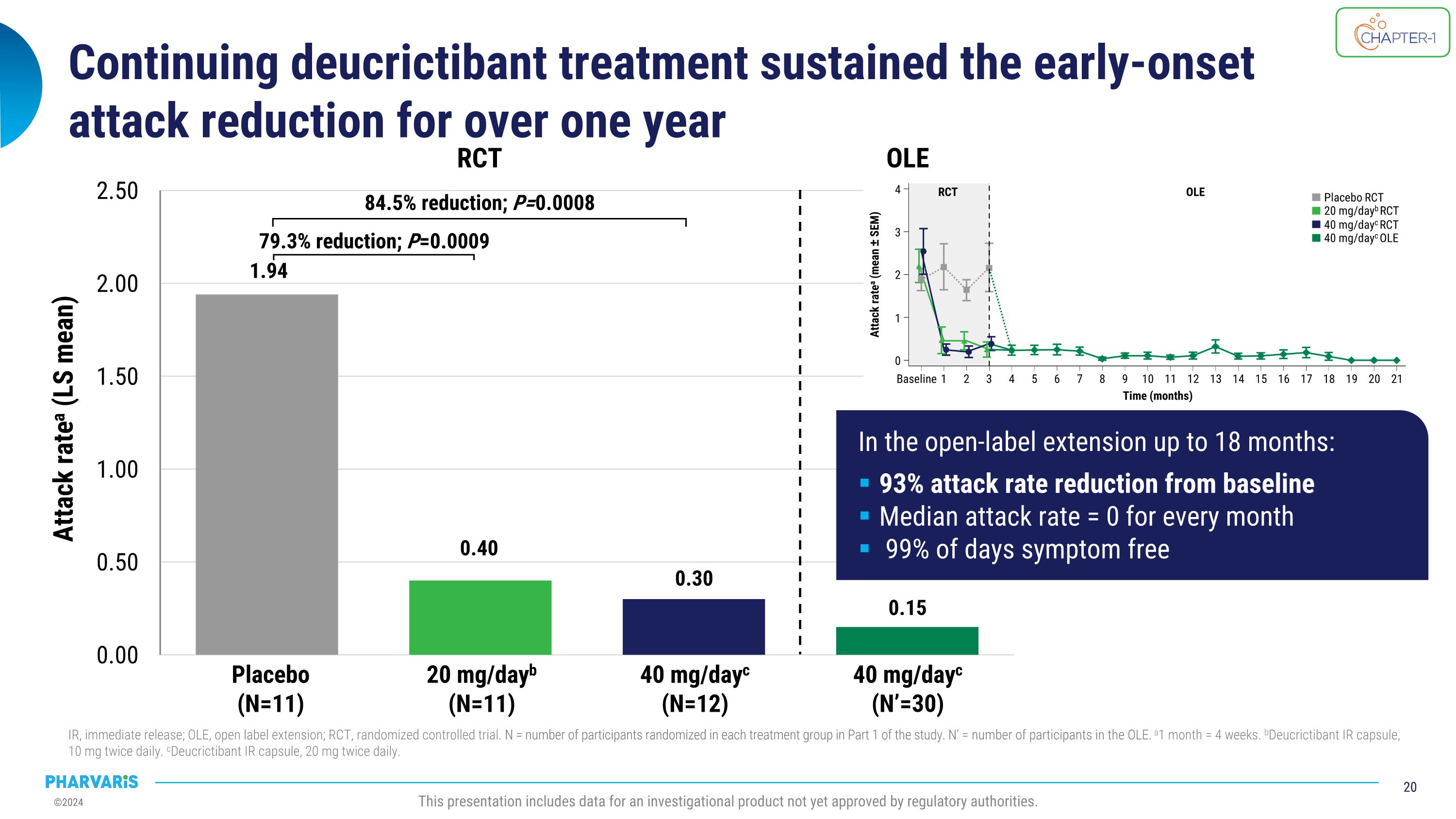
Continuing deucrictibant treatment sustained the early-onset�attack reduction for over one year IR, immediate release; OLE, open label extension; RCT, randomized controlled trial. N = number of participants randomized in each treatment group in Part 1 of the study. N’ = number of participants in the OLE. a1 month = 4 weeks. bDeucrictibant IR capsule, 10 mg twice daily. cDeucrictibant IR capsule, 20 mg twice daily. This presentation includes data for an investigational product not yet approved by regulatory authorities. Placebo (N=11) 20 mg/dayb (N=11) 40 mg/dayc (N=12) RCT 40 mg/dayc (N’=30) OLE 79.3% reduction; P=0.0009 84.5% reduction; P=0.0008 Attack ratea (LS mean) In the open-label extension up to 18 months: 93% attack rate reduction from baseline Median attack rate = 0 for every month 99% of days symptom free
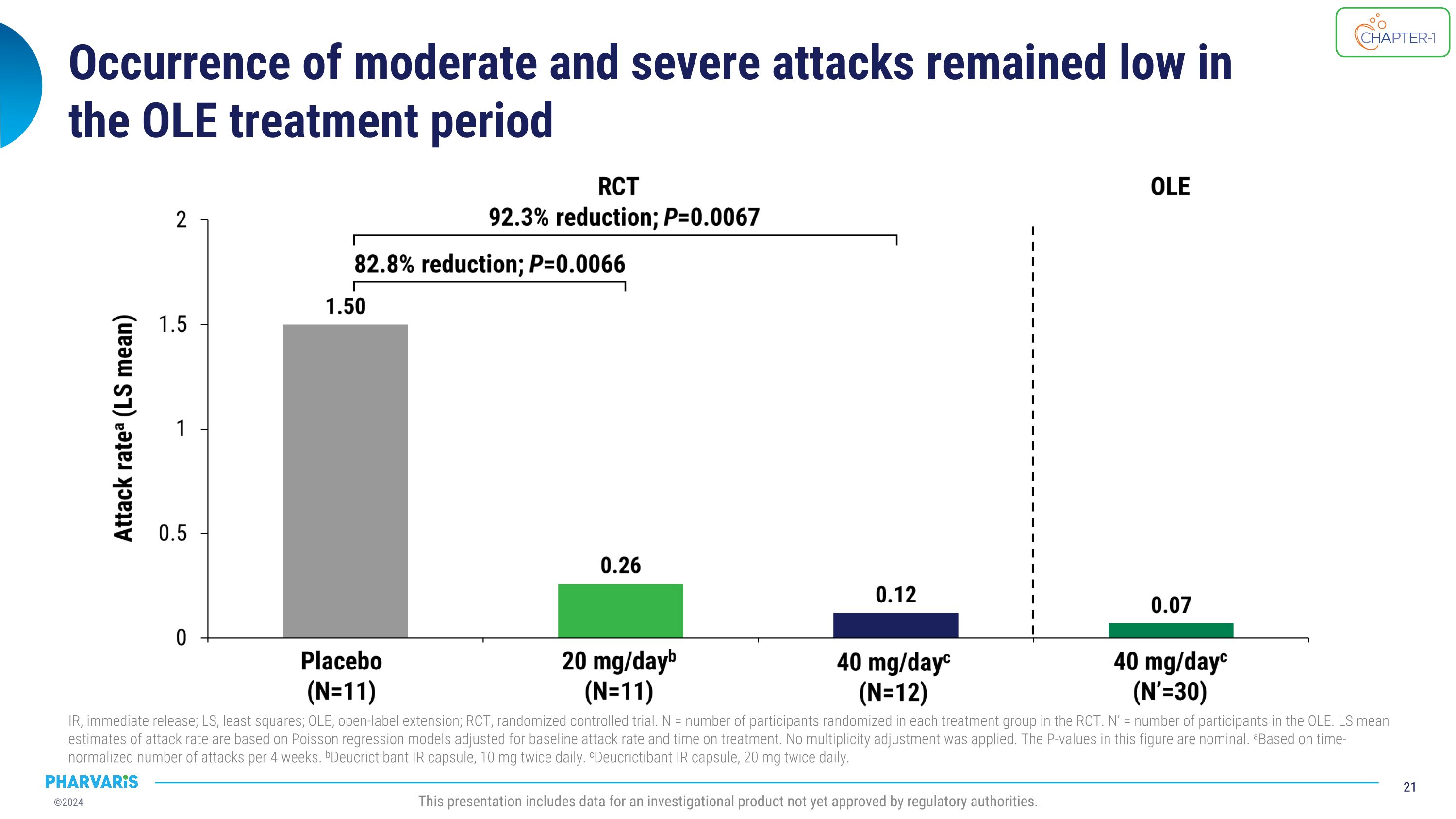
Occurrence of moderate and severe attacks remained low in �the OLE treatment period IR, immediate release; LS, least squares; OLE, open-label extension; RCT, randomized controlled trial. N = number of participants randomized in each treatment group in the RCT. N’ = number of participants in the OLE. LS mean estimates of attack rate are based on Poisson regression models adjusted for baseline attack rate and time on treatment. No multiplicity adjustment was applied. The P-values in this figure are nominal. aBased on time-normalized number of attacks per 4 weeks. bDeucrictibant IR capsule, 10 mg twice daily. cDeucrictibant IR capsule, 20 mg twice daily. This presentation includes data for an investigational product not yet approved by regulatory authorities.
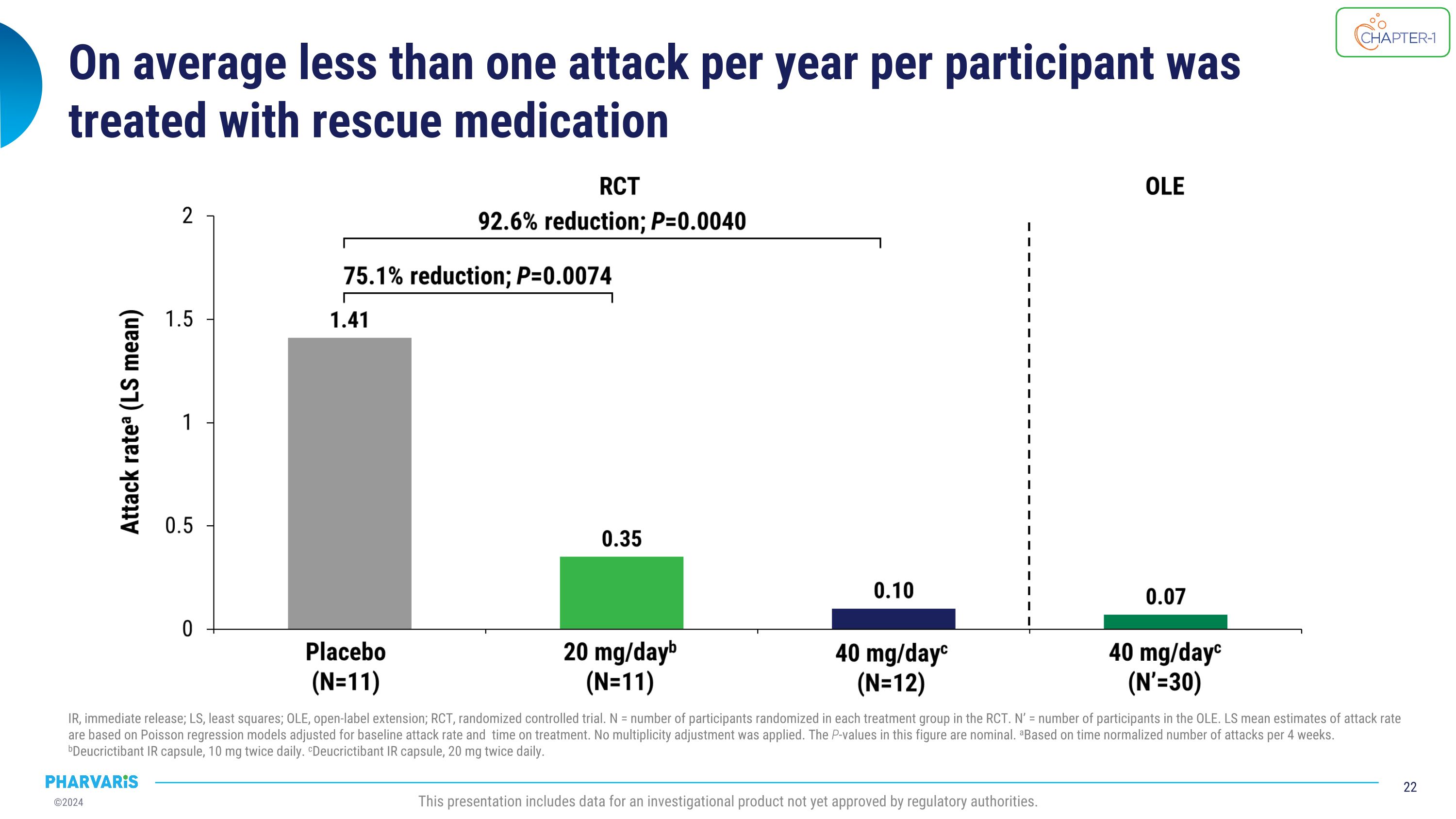
On average less than one attack per year per participant was treated with rescue medication IR, immediate release; LS, least squares; OLE, open-label extension; RCT, randomized controlled trial. N = number of participants randomized in each treatment group in the RCT. N’ = number of participants in the OLE. LS mean estimates of attack rate are based on Poisson regression models adjusted for baseline attack rate and time on treatment. No multiplicity adjustment was applied. The P-values in this figure are nominal. aBased on time normalized number of attacks per 4 weeks. bDeucrictibant IR capsule, 10 mg twice daily. cDeucrictibant IR capsule, 20 mg twice daily. This presentation includes data for an investigational product not yet approved by regulatory authorities.
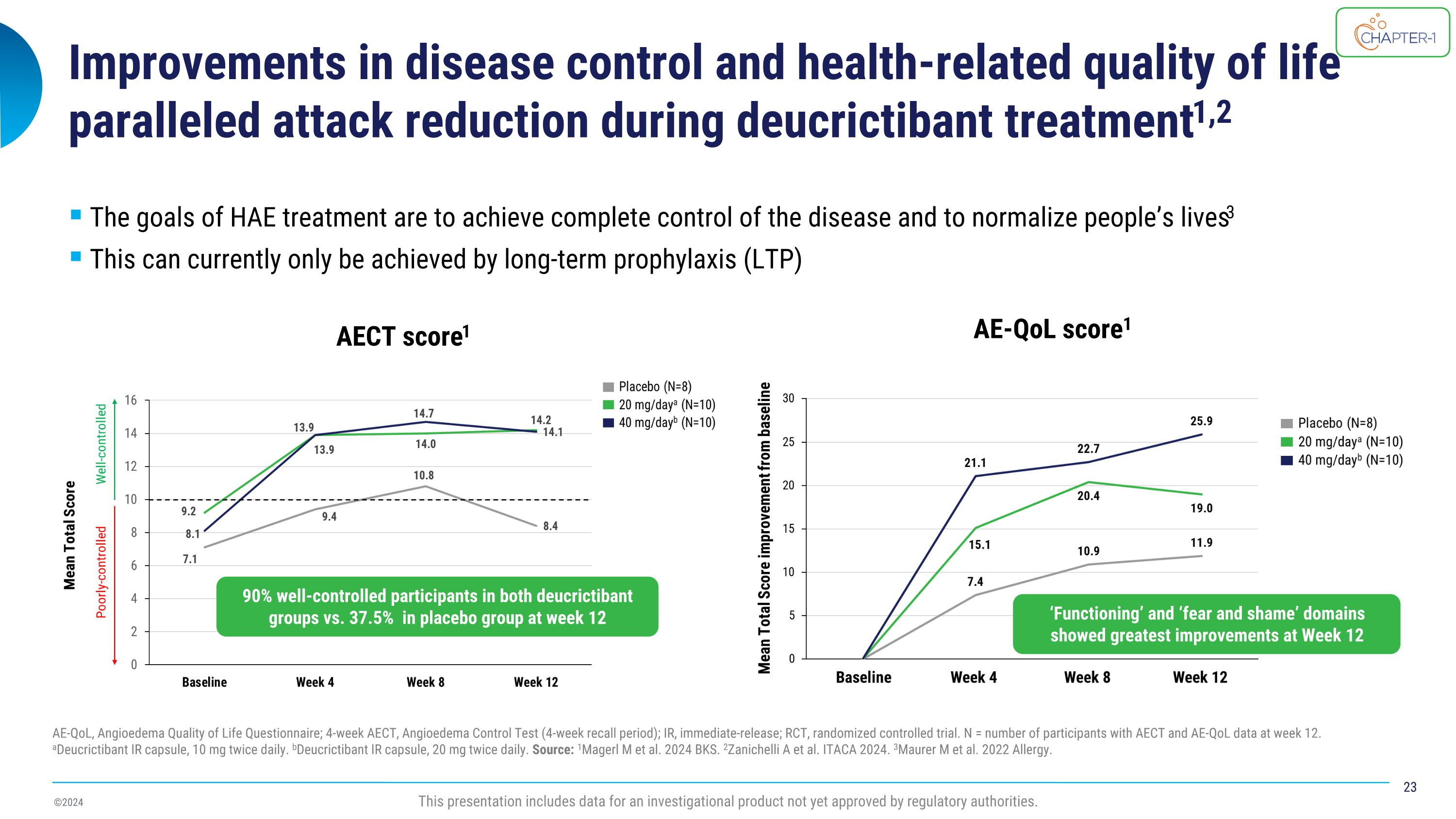
Improvements in disease control and health-related quality of life paralleled attack reduction during deucrictibant treatment1,2 AE-QoL, Angioedema Quality of Life Questionnaire; 4-week AECT, Angioedema Control Test (4-week recall period); IR, immediate-release; RCT, randomized controlled trial. N = number of participants with AECT and AE-QoL data at week 12. aDeucrictibant IR capsule, 10 mg twice daily. bDeucrictibant IR capsule, 20 mg twice daily. Source: 1Magerl M et al. 2024 BKS. 2Zanichelli A et al. ITACA 2024. 3Maurer M et al. 2022 Allergy. The goals of HAE treatment are to achieve complete control of the disease and to normalize people’s lives3 This can currently only be achieved by long-term prophylaxis (LTP) AECT score1 AE-QoL score1 This presentation includes data for an investigational product not yet approved by regulatory authorities. 90% well-controlled participants in both deucrictibant groups vs. 37.5% in placebo group at week 12 ‘Functioning’ and ‘fear and shame’ domains showed greatest improvements at Week 12
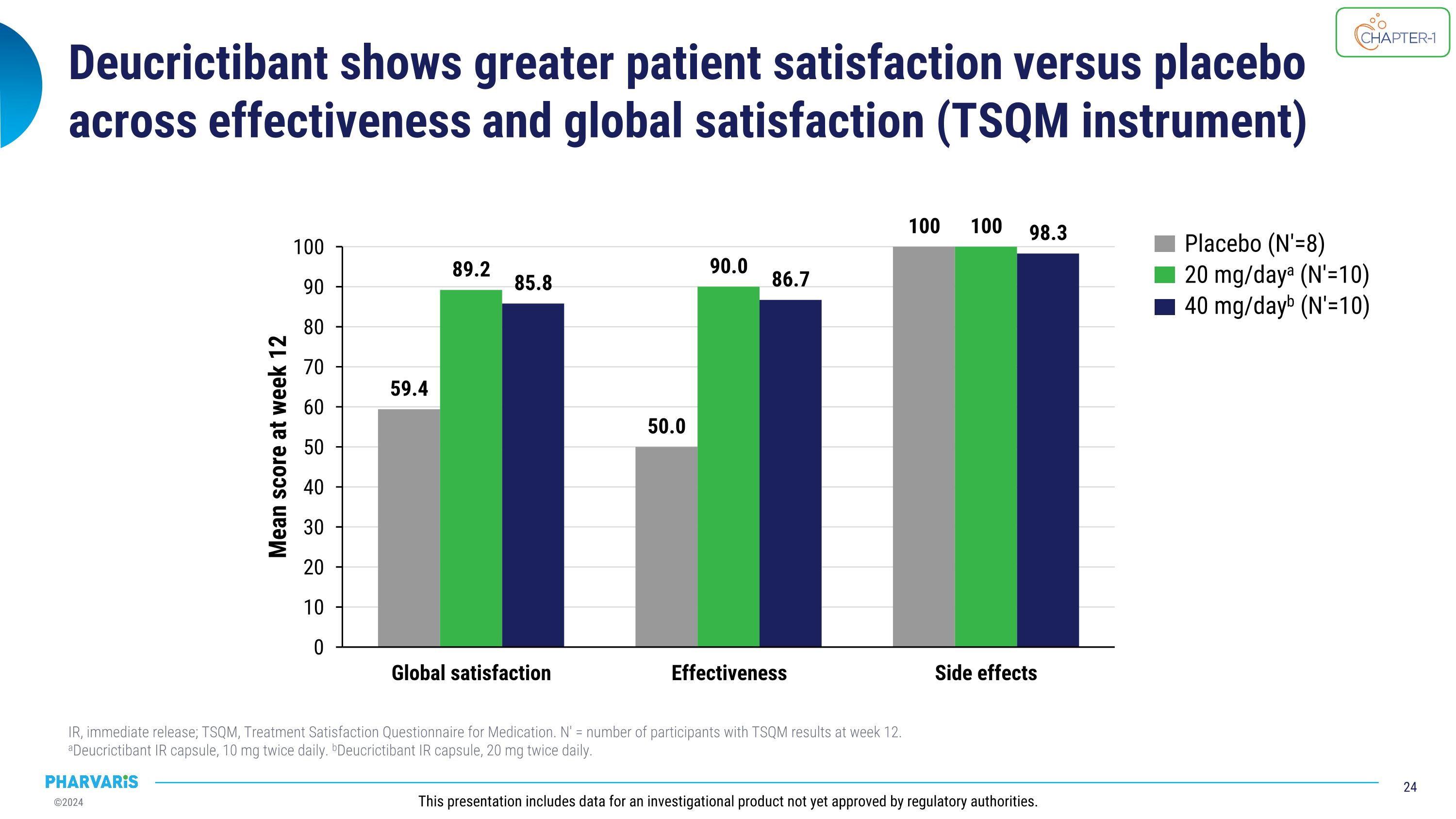
Deucrictibant shows greater patient satisfaction versus placebo across effectiveness and global satisfaction (TSQM instrument) IR, immediate release; TSQM, Treatment Satisfaction Questionnaire for Medication. N′ = number of participants with TSQM results at week 12. �aDeucrictibant IR capsule, 10 mg twice daily. bDeucrictibant IR capsule, 20 mg twice daily. This presentation includes data for an investigational product not yet approved by regulatory authorities. Placebo (N′=8) 20 mg/daya (N′=10) 40 mg/dayb (N′=10)
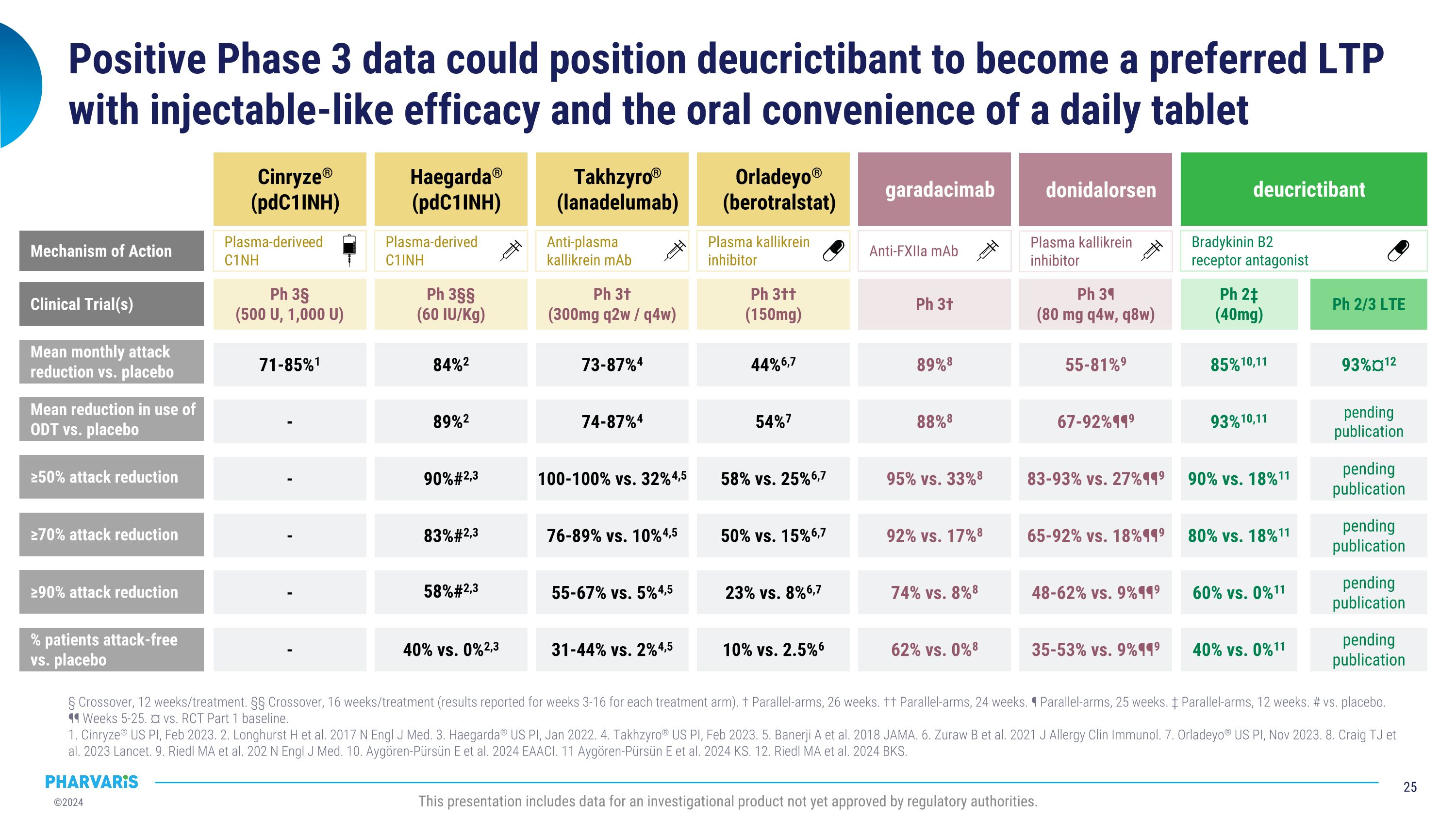
Positive Phase 3 data could position deucrictibant to become a preferred LTP with injectable-like efficacy and the oral convenience of a daily tablet § Crossover, 12 weeks/treatment. §§ Crossover, 16 weeks/treatment (results reported for weeks 3-16 for each treatment arm). † Parallel-arms, 26 weeks. †† Parallel-arms, 24 weeks. ¶ Parallel-arms, 25 weeks. ‡ Parallel-arms, 12 weeks. # vs. placebo. �¶¶ Weeks 5-25. ¤ vs. RCT Part 1 baseline. 1. Cinryze® US PI, Feb 2023. 2. Longhurst H et al. 2017 N Engl J Med. 3. Haegarda® US PI, Jan 2022. 4. Takhzyro® US PI, Feb 2023. 5. Banerji A et al. 2018 JAMA. 6. Zuraw B et al. 2021 J Allergy Clin Immunol. 7. Orladeyo® US PI, Nov 2023. 8. Craig TJ et al. 2023 Lancet. 9. Riedl MA et al. 202 N Engl J Med. 10. Aygören-Pürsün E et al. 2024 EAACI. 11 Aygören-Pürsün E et al. 2024 KS. 12. Riedl MA et al. 2024 BKS. This presentation includes data for an investigational product not yet approved by regulatory authorities. Mean monthly attack reduction vs. placebo ≥50% attack reduction ≥70% attack reduction Cinryze® (pdC1INH) Haegarda® (pdC1INH) Takhzyro® (lanadelumab) Orladeyo®�(berotralstat) garadacimab 89%8 95% vs. 33%8 92% vs. 17%8 74% vs. 8%8 62% vs. 0%8 71-85%1 - - - - 84%2 90%#2,3 83%#2,3 58%#2,3 40% vs. 0%2,3 73-87%4 100-100% vs. 32%4,5 76-89% vs. 10%4,5 55-67% vs. 5%4,5 31-44% vs. 2%4,5 44%6,7 58% vs. 25%6,7 50% vs. 15%6,7 23% vs. 8%6,7 10% vs. 2.5%6 ≥90% attack reduction % patients attack-free vs. placebo Anti-FXIIa mAb Plasma kallikrein�inhibitor Anti-plasma�kallikrein mAb Plasma-derived�C1INH Plasma-deriveed�C1NH Mechanism of Action donidalorsen 55-81%9 83-93% vs. 27%¶¶9 65-92% vs. 18%¶¶9 48-62% vs. 9%¶¶9 35-53% vs. 9%¶¶9 Plasma kallikrein�inhibitor deucrictibant Ph 3† Ph 3†† (150mg) Ph 3† (300mg q2w / q4w) Ph 3§§ (60 IU/Kg) Ph 3§ (500 U, 1,000 U) Clinical Trial(s) Ph 3¶ (80 mg q4w, q8w) Ph 2‡ (40mg) Ph 2/3 LTE Bradykinin B2�receptor antagonist 85%10,11 90% vs. 18%11 80% vs. 18%11 60% vs. 0%11 40% vs. 0%11 93%¤12 pending publication pending publication pending publication pending publication Mean reduction in use of ODT vs. placebo 88%8 - 89%2 74-87%4 54%7 67-92%¶¶9 93%10,11 pending publication

CHAPTER-3 and CHAPTER-4 Clinical Studies
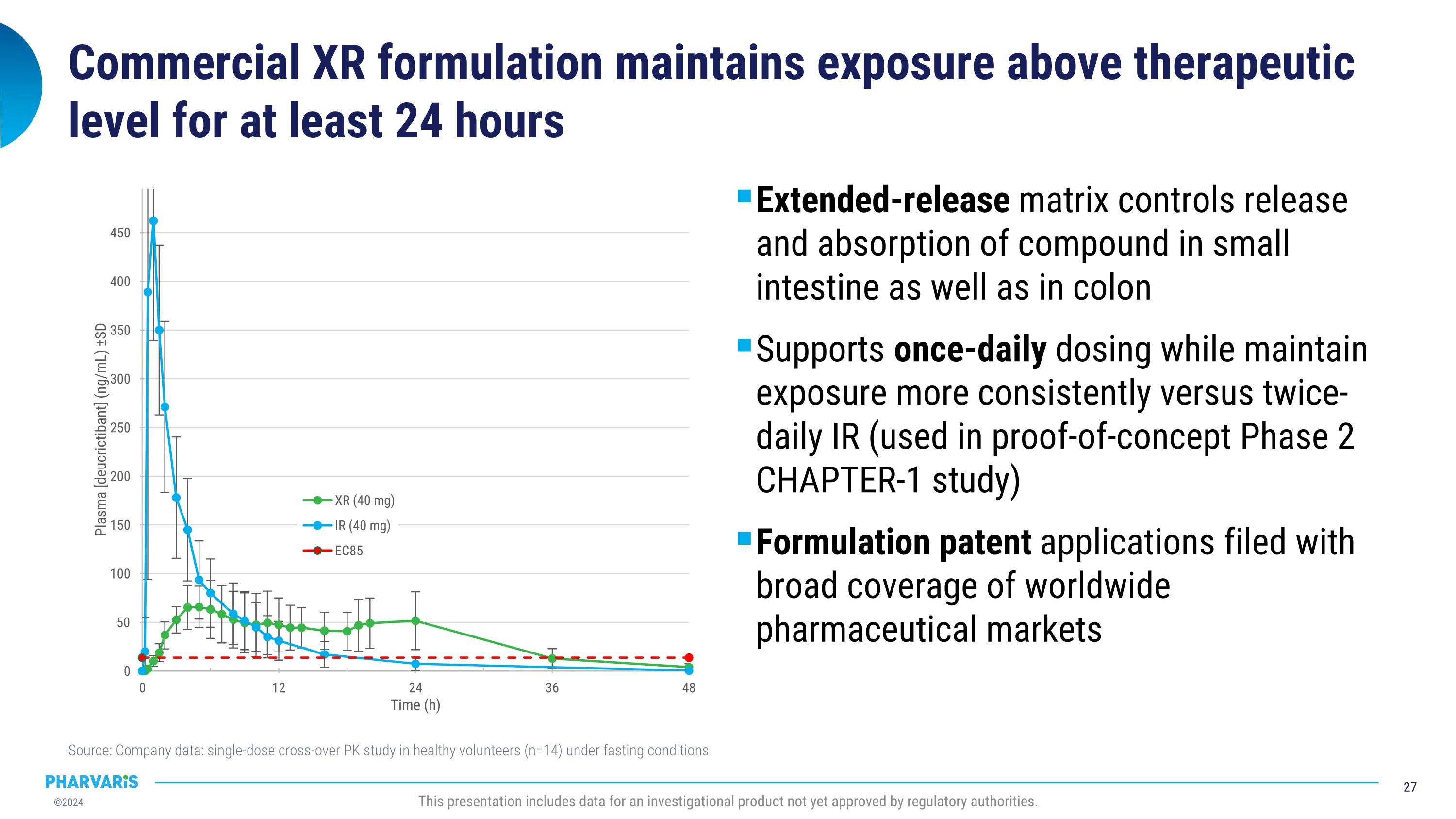
Commercial XR formulation maintains exposure above therapeutic level for at least 24 hours Extended-release matrix controls release and absorption of compound in small intestine as well as in colon Supports once-daily dosing while maintain exposure more consistently versus twice-daily IR (used in proof-of-concept Phase 2 CHAPTER-1 study) Formulation patent applications filed with broad coverage of worldwide pharmaceutical markets Source: Company data: single-dose cross-over PK study in healthy volunteers (n=14) under fasting conditions This presentation includes data for an investigational product not yet approved by regulatory authorities.
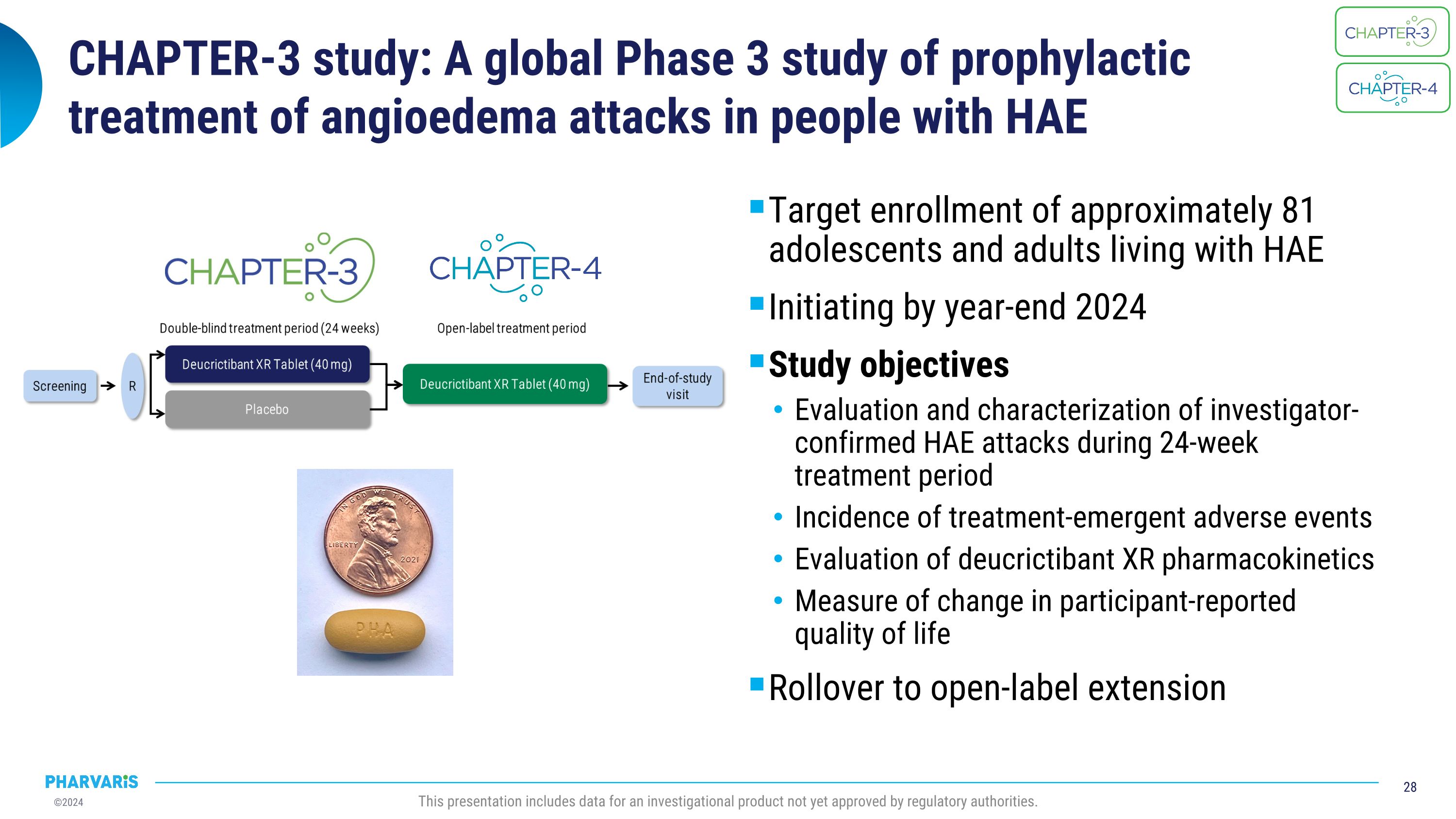
CHAPTER-3 study: A global Phase 3 study of prophylactic �treatment of angioedema attacks in people with HAE Target enrollment of approximately 81 adolescents and adults living with HAE Initiating by year-end 2024 Study objectives Evaluation and characterization of investigator-confirmed HAE attacks during 24-week treatment period Incidence of treatment-emergent adverse events Evaluation of deucrictibant XR pharmacokinetics Measure of change in participant-reported quality of life Rollover to open-label extension This presentation includes data for an investigational product not yet approved by regulatory authorities.
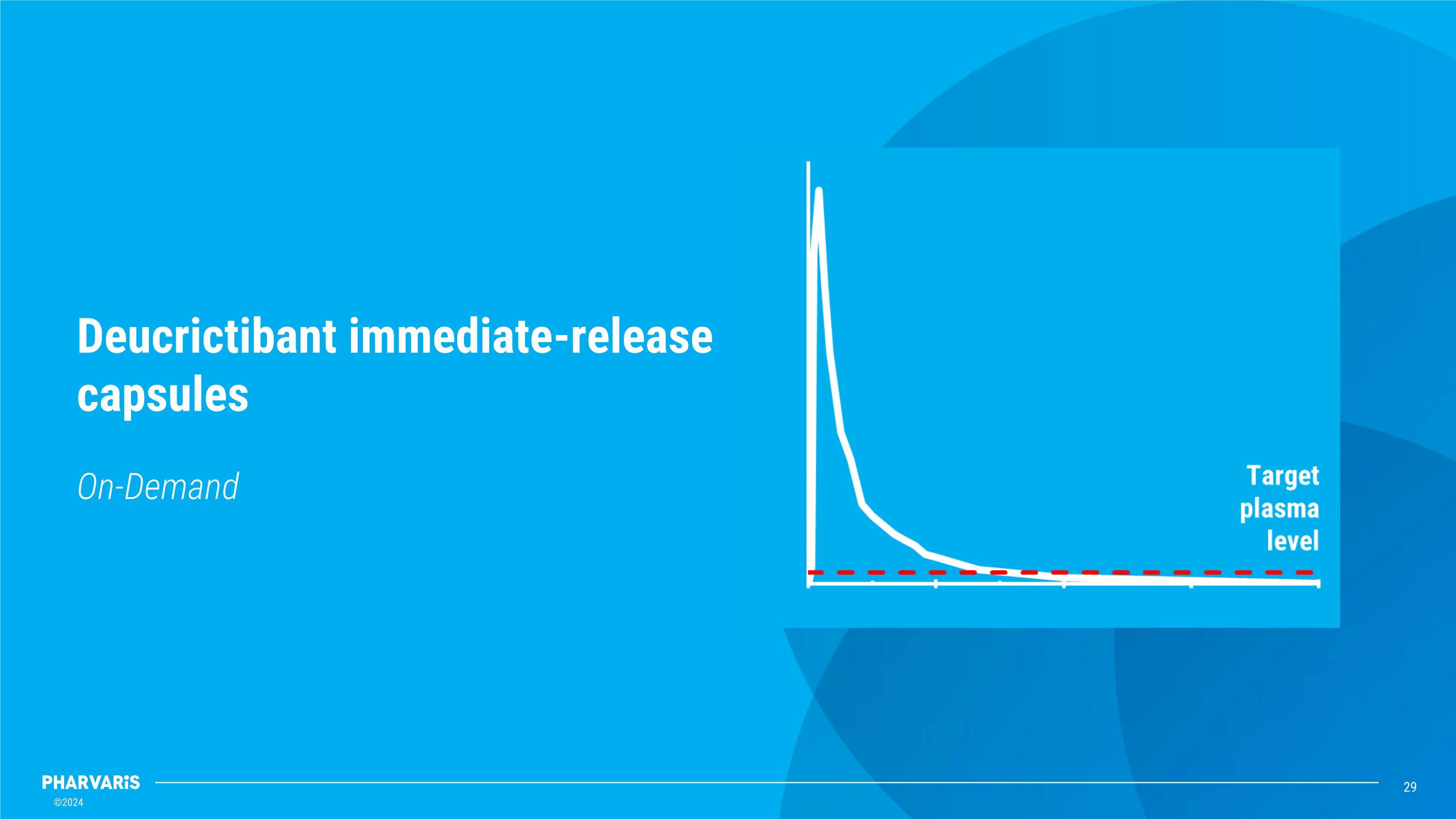
On-Demand Deucrictibant immediate-release capsules
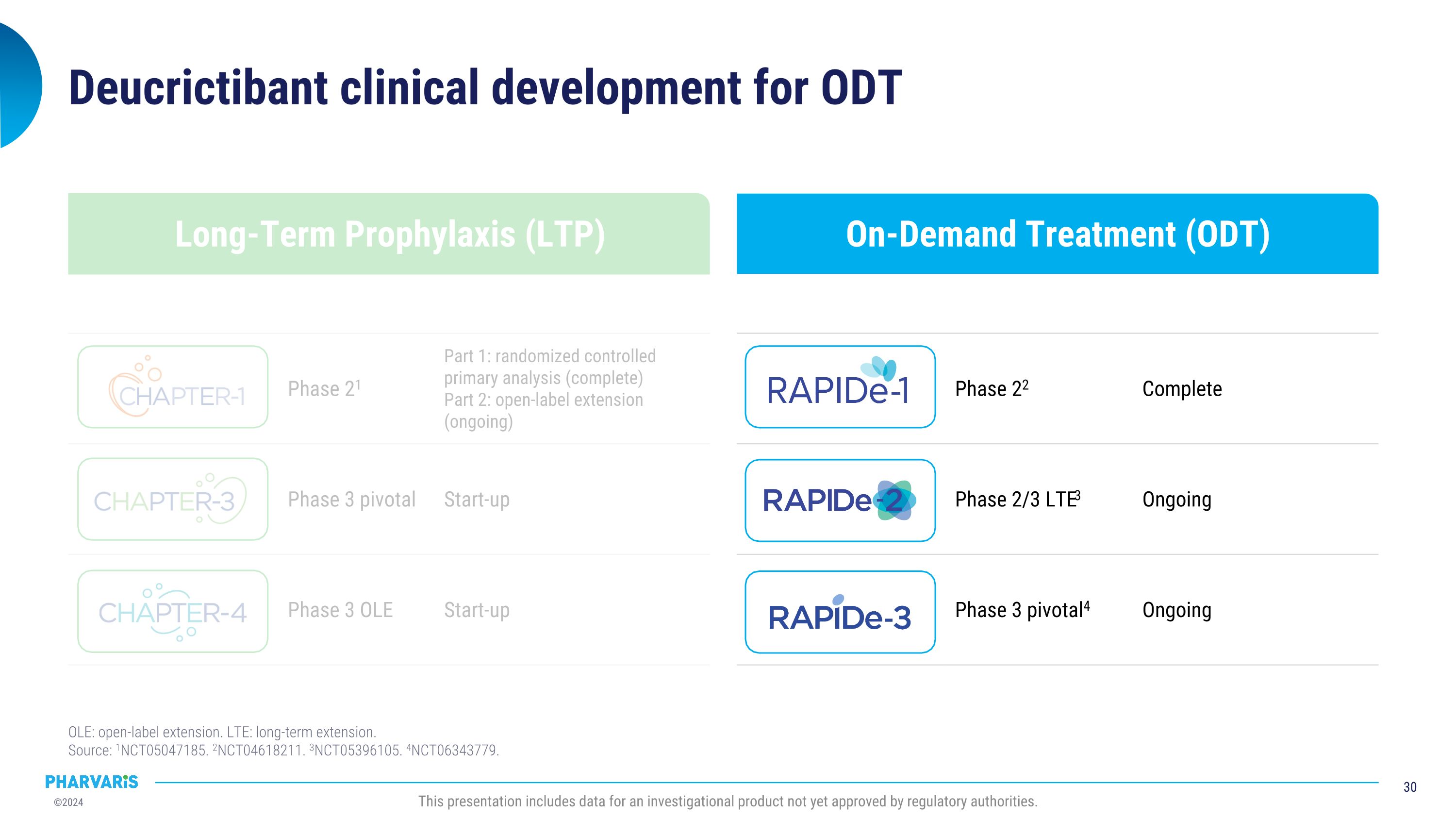
On-Demand Treatment (ODT) Long-Term Prophylaxis (LTP) Deucrictibant clinical development for ODT OLE: open-label extension. LTE: long-term extension.�Source: 1NCT05047185. 2NCT04618211. 3NCT05396105. 4NCT06343779. Phase 22 Complete Phase 2/3 LTE3 Ongoing Phase 3 pivotal4 Ongoing Phase 21 Part 1: randomized controlled primary analysis (complete) Part 2: open-label extension (ongoing) Phase 3 pivotal Start-up Phase 3 OLE Start-up This presentation includes data for an investigational product not yet approved by regulatory authorities.
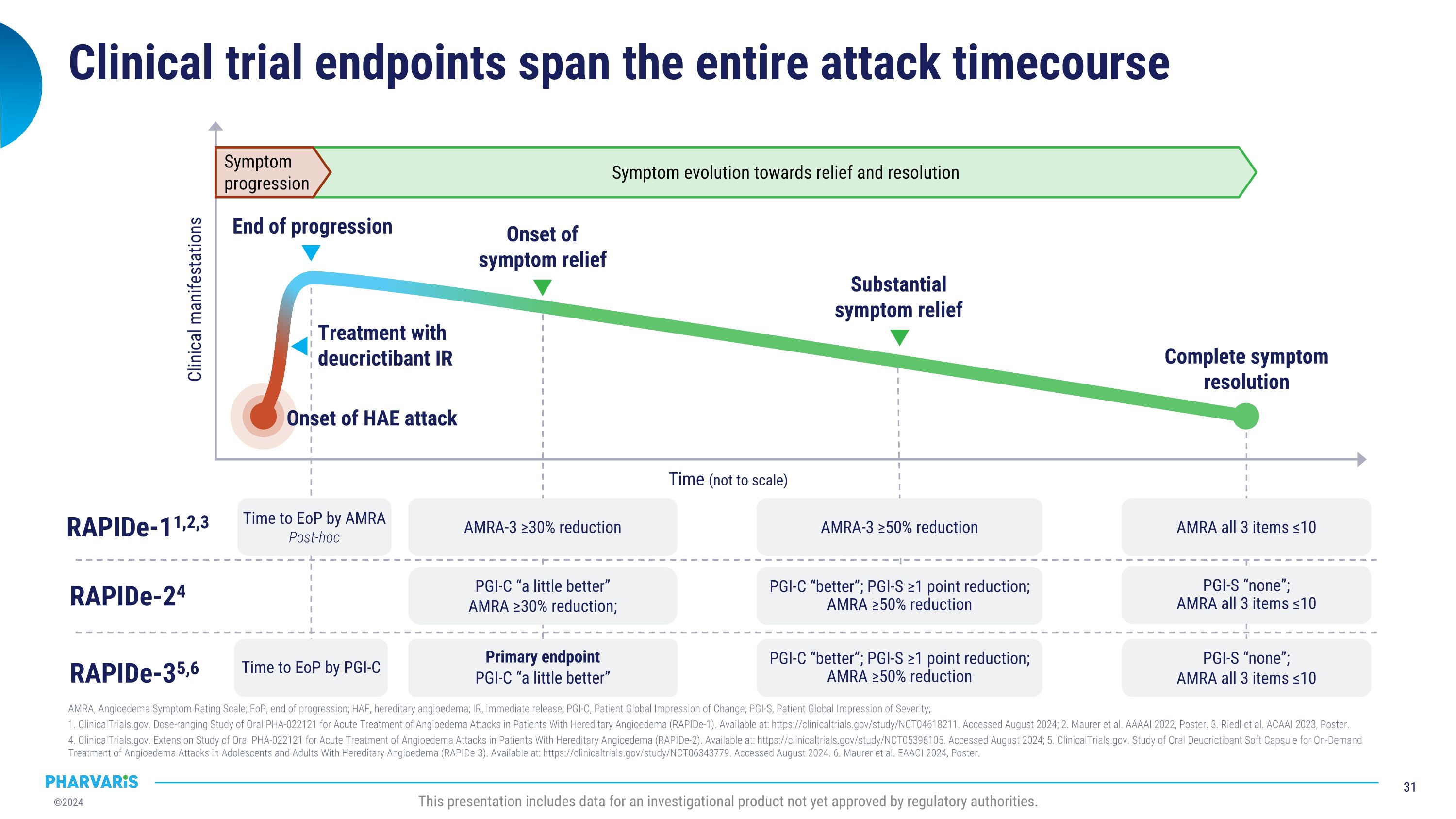
Symptom evolution towards relief and resolution Symptom progression Clinical trial endpoints span the entire attack timecourse AMRA, Angioedema Symptom Rating Scale; EoP, end of progression; HAE, hereditary angioedema; IR, immediate release; PGI-C, Patient Global Impression of Change; PGI-S, Patient Global Impression of Severity; 1. ClinicalTrials.gov. Dose-ranging Study of Oral PHA-022121 for Acute Treatment of Angioedema Attacks in Patients With Hereditary Angioedema (RAPIDe-1). Available at: https://clinicaltrials.gov/study/NCT04618211. Accessed August 2024; 2. Maurer et al. AAAAI 2022, Poster. 3. Riedl et al. ACAAI 2023, Poster. 4. ClinicalTrials.gov. Extension Study of Oral PHA-022121 for Acute Treatment of Angioedema Attacks in Patients With Hereditary Angioedema (RAPIDe-2). Available at: https://clinicaltrials.gov/study/NCT05396105. Accessed August 2024; 5. ClinicalTrials.gov. Study of Oral Deucrictibant Soft Capsule for On-Demand Treatment of Angioedema Attacks in Adolescents and Adults With Hereditary Angioedema (RAPIDe-3). Available at: https://clinicaltrials.gov/study/NCT06343779. Accessed August 2024. 6. Maurer et al. EAACI 2024, Poster. This presentation includes data for an investigational product not yet approved by regulatory authorities. Time (not to scale) Clinical manifestations Treatment with deucrictibant IR End of progression Onset of symptom relief Complete symptom resolution RAPIDe-11,2,3 RAPIDe-35,6 AMRA all 3 items ≤10 PGI-S “none”; AMRA all 3 items ≤10 Time to EoP by PGI-C Onset of HAE attack AMRA-3 ≥30% reduction Primary endpoint PGI-C “a little better” Substantial symptom relief AMRA-3 ≥50% reduction RAPIDe-24 PGI-S “none”; AMRA all 3 items ≤10 PGI-C “a little better” AMRA ≥30% reduction; PGI-C “better”; PGI-S ≥1 point reduction; AMRA ≥50% reduction PGI-C “better”; PGI-S ≥1 point reduction; AMRA ≥50% reduction Time to EoP by AMRA Post-hoc
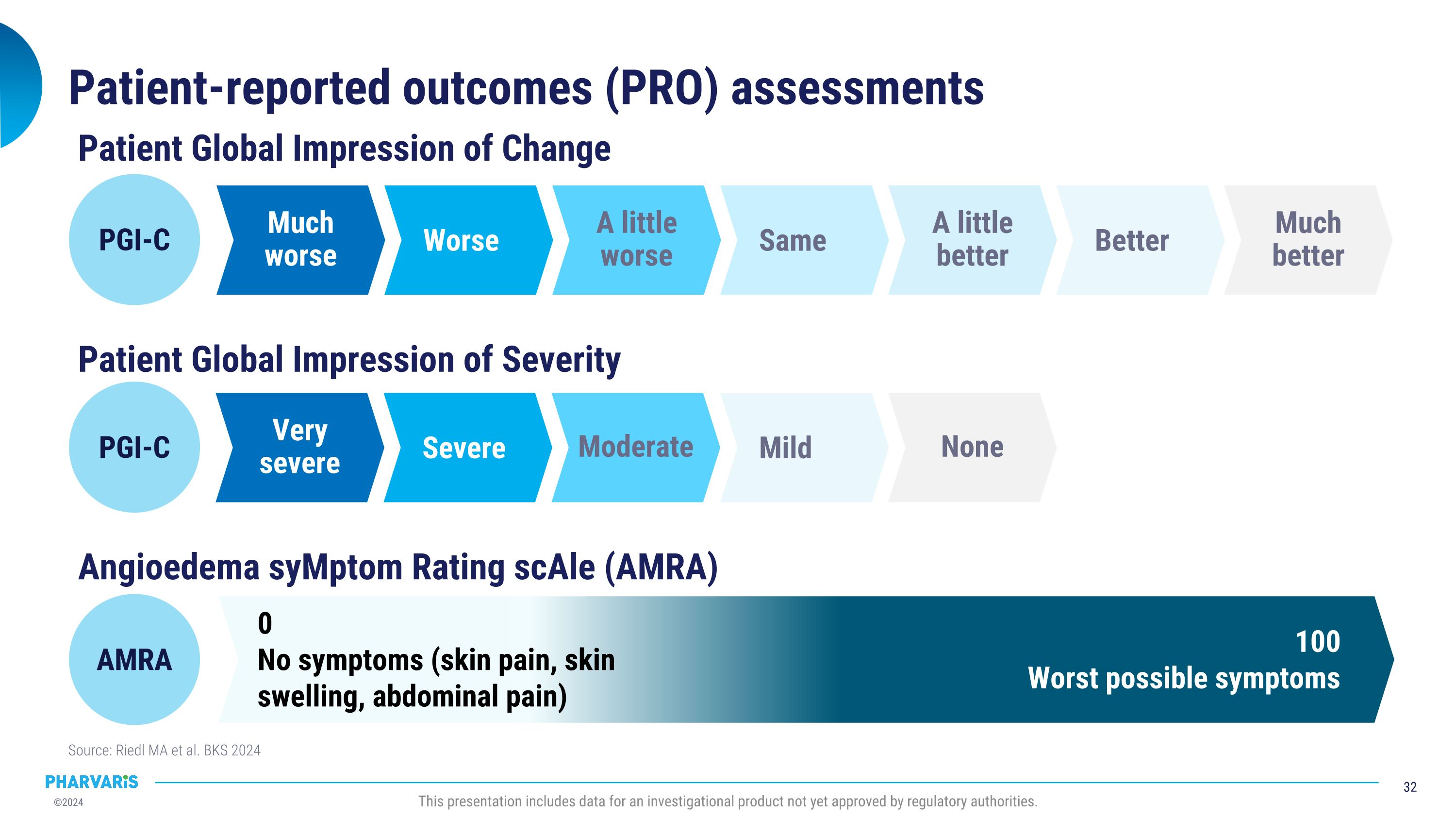
Patient-reported outcomes (PRO) assessments Source: Riedl MA et al. BKS 2024 This presentation includes data for an investigational product not yet approved by regulatory authorities. Patient Global Impression of Change PGI-C Much worse Worse A little worse Same A little better Better Much better Patient Global Impression of Severity Very severe Severe Moderate Mild None PGI-C Angioedema syMptom Rating scAle (AMRA) 100 Worst possible symptoms 0 No symptoms (skin pain, skin�swelling, abdominal pain) AMRA
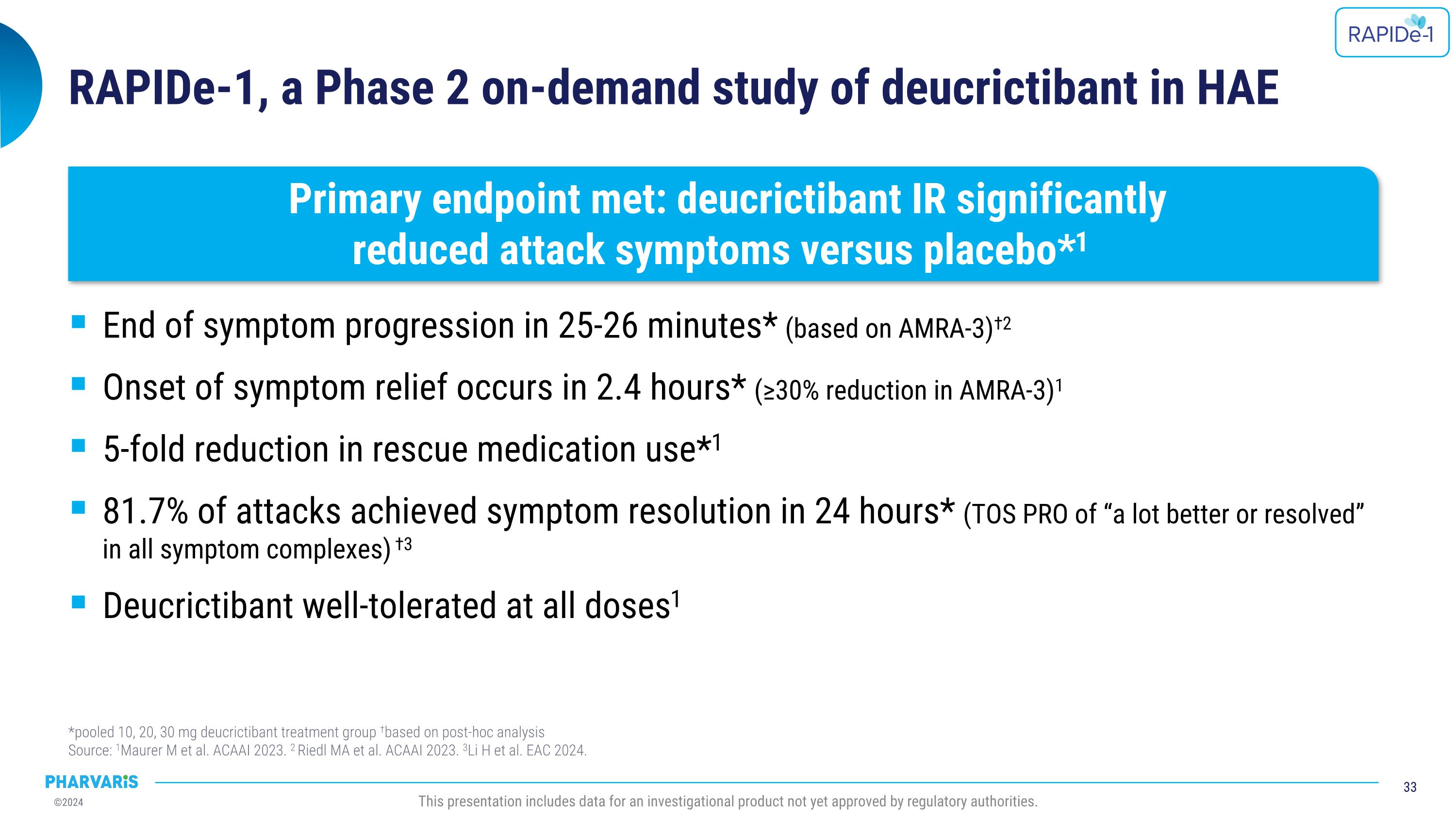
RAPIDe-1, a Phase 2 on-demand study of deucrictibant in HAE End of symptom progression in 25-26 minutes* (based on AMRA-3)†2 Onset of symptom relief occurs in 2.4 hours* (≥30% reduction in AMRA-3)1 5-fold reduction in rescue medication use*1 81.7% of attacks achieved symptom resolution in 24 hours* (TOS PRO of “a lot better or resolved” in all symptom complexes) †3 Deucrictibant well-tolerated at all doses1 *pooled 10, 20, 30 mg deucrictibant treatment group †based on post-hoc analysis Source: 1Maurer M et al. ACAAI 2023. 2 Riedl MA et al. ACAAI 2023. 3Li H et al. EAC 2024. Primary endpoint met: deucrictibant IR significantly�reduced attack symptoms versus placebo*1 This presentation includes data for an investigational product not yet approved by regulatory authorities.
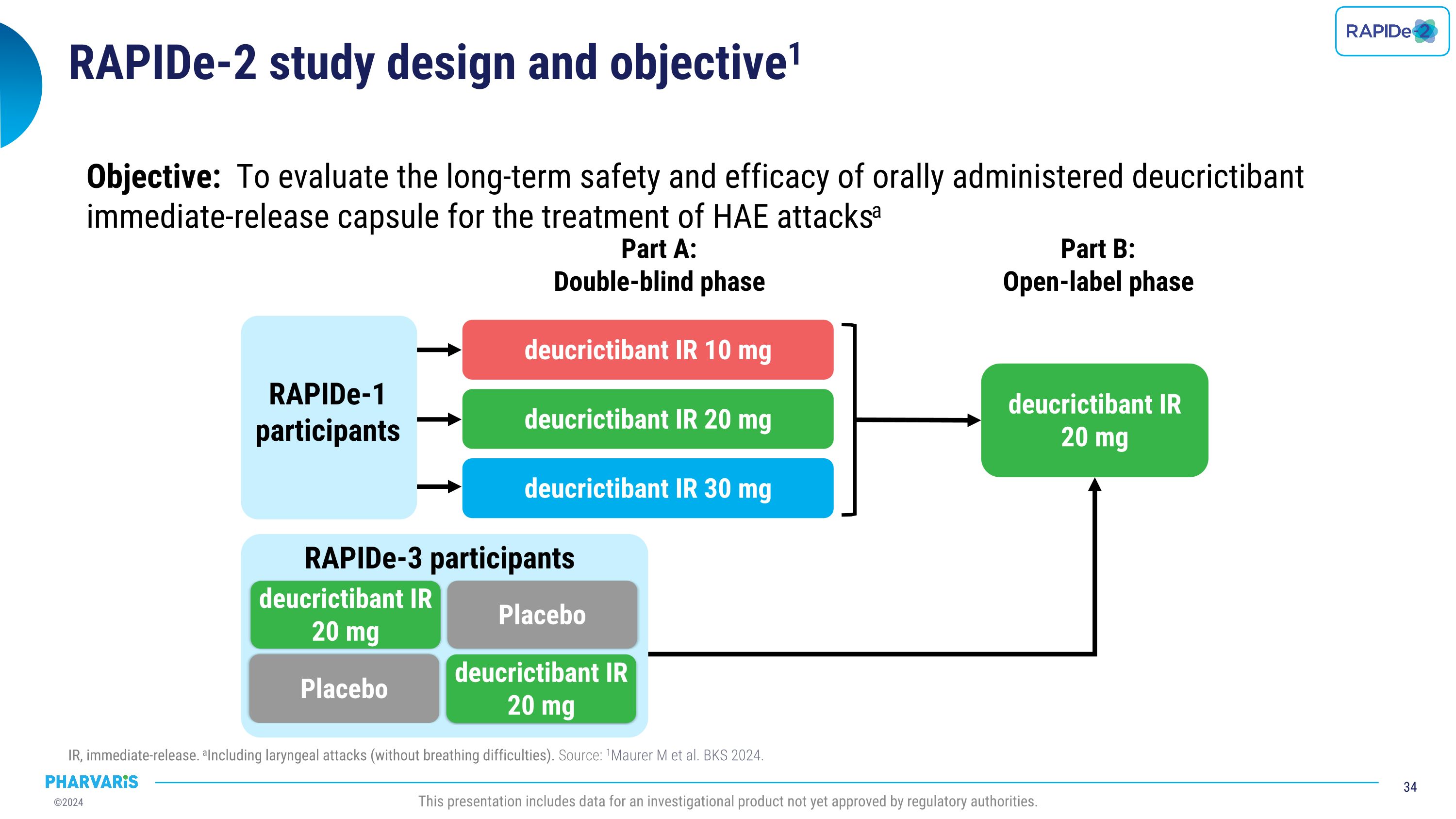
RAPIDe-2 study design and objective1 IR, immediate-release. aIncluding laryngeal attacks (without breathing difficulties). Source: 1Maurer M et al. BKS 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. Objective: To evaluate the long-term safety and efficacy of orally administered deucrictibant immediate-release capsule for the treatment of HAE attacksa Part A: Double-blind phase Part B: Open-label phase RAPIDe-1 participants deucrictibant IR 10 mg deucrictibant IR 20 mg deucrictibant IR 30 mg deucrictibant IR �20 mg RAPIDe-3 participants Placebo deucrictibant IR�20 mg Placebo deucrictibant IR�20 mg
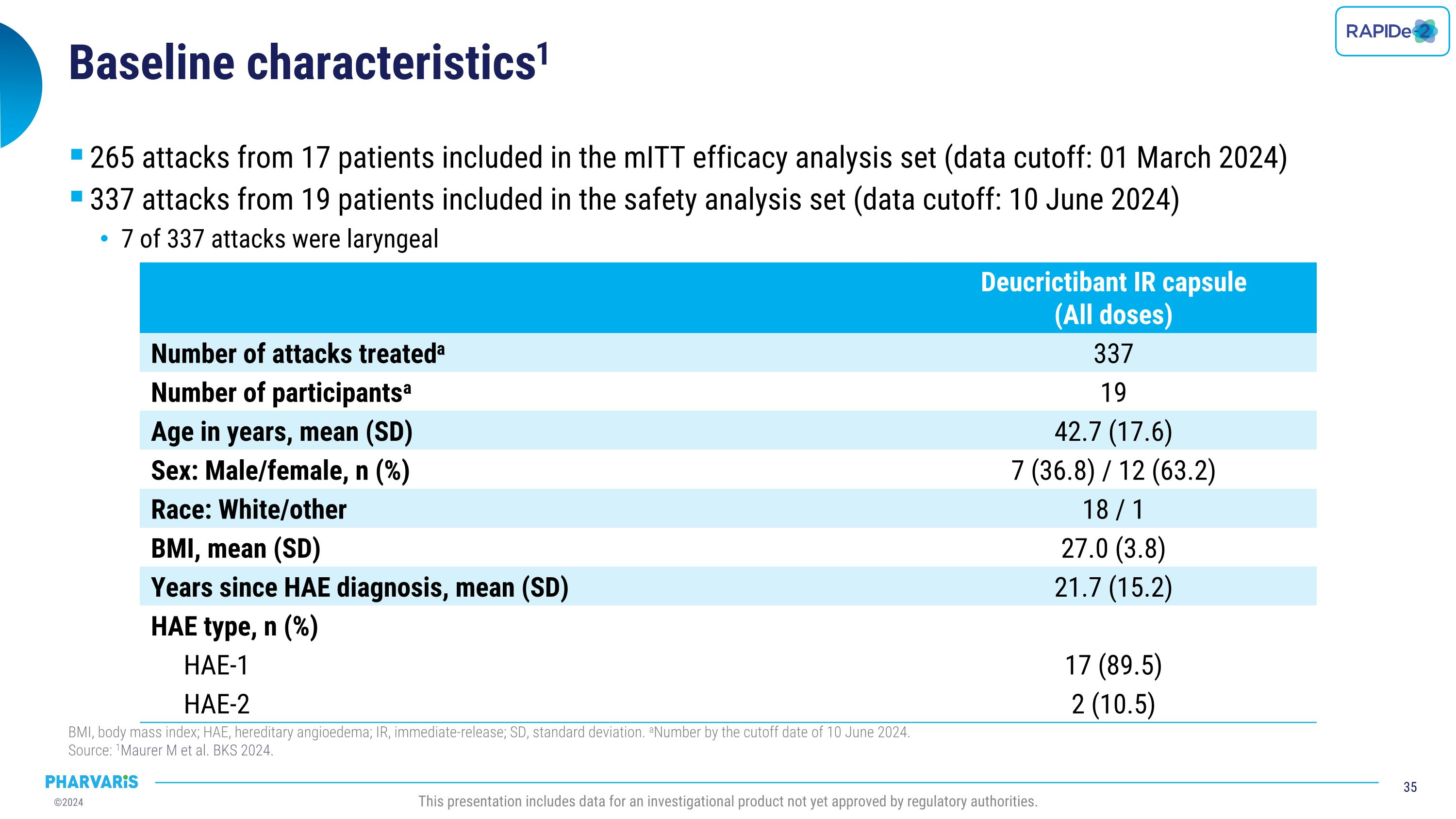
Baseline characteristics1 265 attacks from 17 patients included in the mITT efficacy analysis set (data cutoff: 01 March 2024) 337 attacks from 19 patients included in the safety analysis set (data cutoff: 10 June 2024) 7 of 337 attacks were laryngeal BMI, body mass index; HAE, hereditary angioedema; IR, immediate-release; SD, standard deviation. aNumber by the cutoff date of 10 June 2024.�Source: 1Maurer M et al. BKS 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. Deucrictibant IR capsule (All doses) Number of attacks treateda 337 Number of participantsa 19 Age in years, mean (SD) 42.7 (17.6) Sex: Male/female, n (%) 7 (36.8) / 12 (63.2) Race: White/other 18 / 1 BMI, mean (SD) 27.0 (3.8) Years since HAE diagnosis, mean (SD) 21.7 (15.2) HAE type, n (%) HAE-1 17 (89.5) HAE-2 2 (10.5)
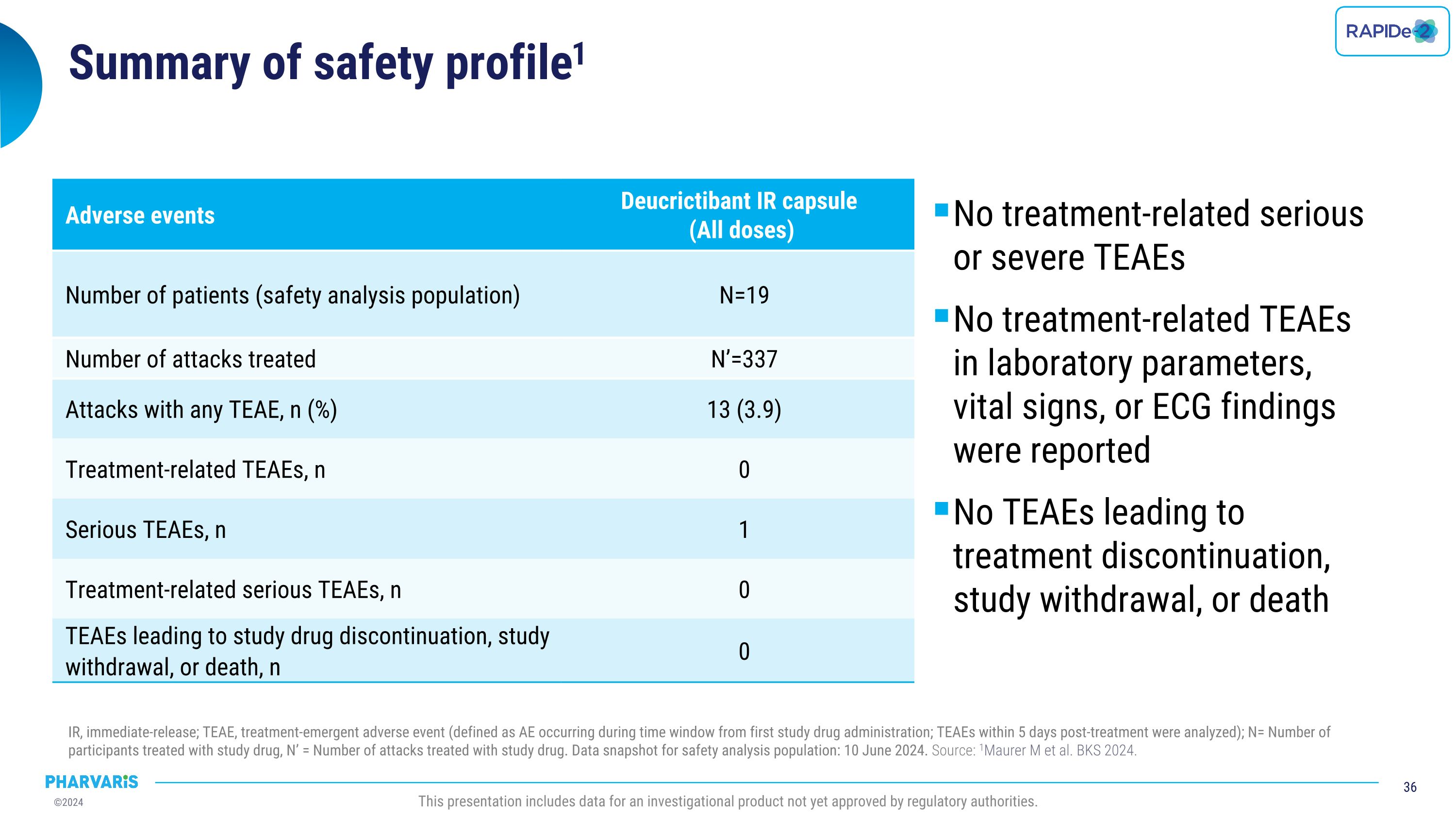
Summary of safety profile1 No treatment-related serious or severe TEAEs No treatment-related TEAEs in laboratory parameters, vital signs, or ECG findings were reported No TEAEs leading to treatment discontinuation, study withdrawal, or death IR, immediate-release; TEAE, treatment-emergent adverse event (defined as AE occurring during time window from first study drug administration; TEAEs within 5 days post-treatment were analyzed); N= Number of participants treated with study drug, N’ = Number of attacks treated with study drug. Data snapshot for safety analysis population: 10 June 2024. Source: 1Maurer M et al. BKS 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. Adverse events Deucrictibant IR capsule� (All doses) Number of patients (safety analysis population) N=19 Number of attacks treated N’=337 Attacks with any TEAE, n (%) 13 (3.9) Treatment-related TEAEs, n 0 Serious TEAEs, n 1 Treatment-related serious TEAEs, n 0 TEAEs leading to study drug discontinuation, study withdrawal, or death, n 0
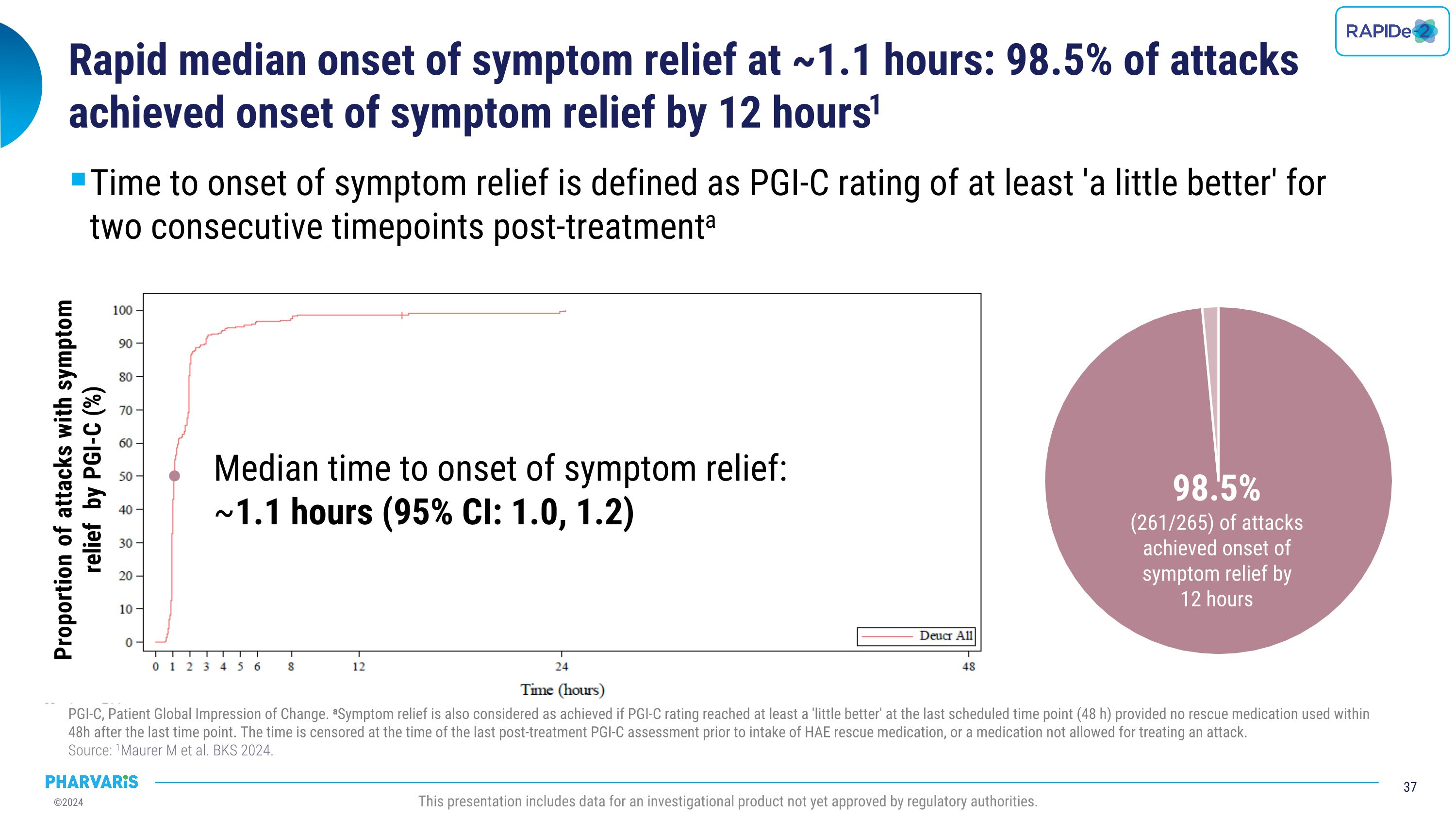
Rapid median onset of symptom relief at ~1.1 hours: 98.5% of attacks achieved onset of symptom relief by 12 hours1 Time to onset of symptom relief is defined as PGI-C rating of at least 'a little better' for two consecutive timepoints post-treatmenta PGI-C, Patient Global Impression of Change. aSymptom relief is also considered as achieved if PGI-C rating reached at least a 'little better' at the last scheduled time point (48 h) provided no rescue medication used within 48h after the last time point. The time is censored at the time of the last post-treatment PGI-C assessment prior to intake of HAE rescue medication, or a medication not allowed for treating an attack.�Source: 1Maurer M et al. BKS 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. Proportion of attacks with symptom relief by PGI-C (%) Median time to onset of symptom relief:�~1.1 hours (95% CI: 1.0, 1.2)
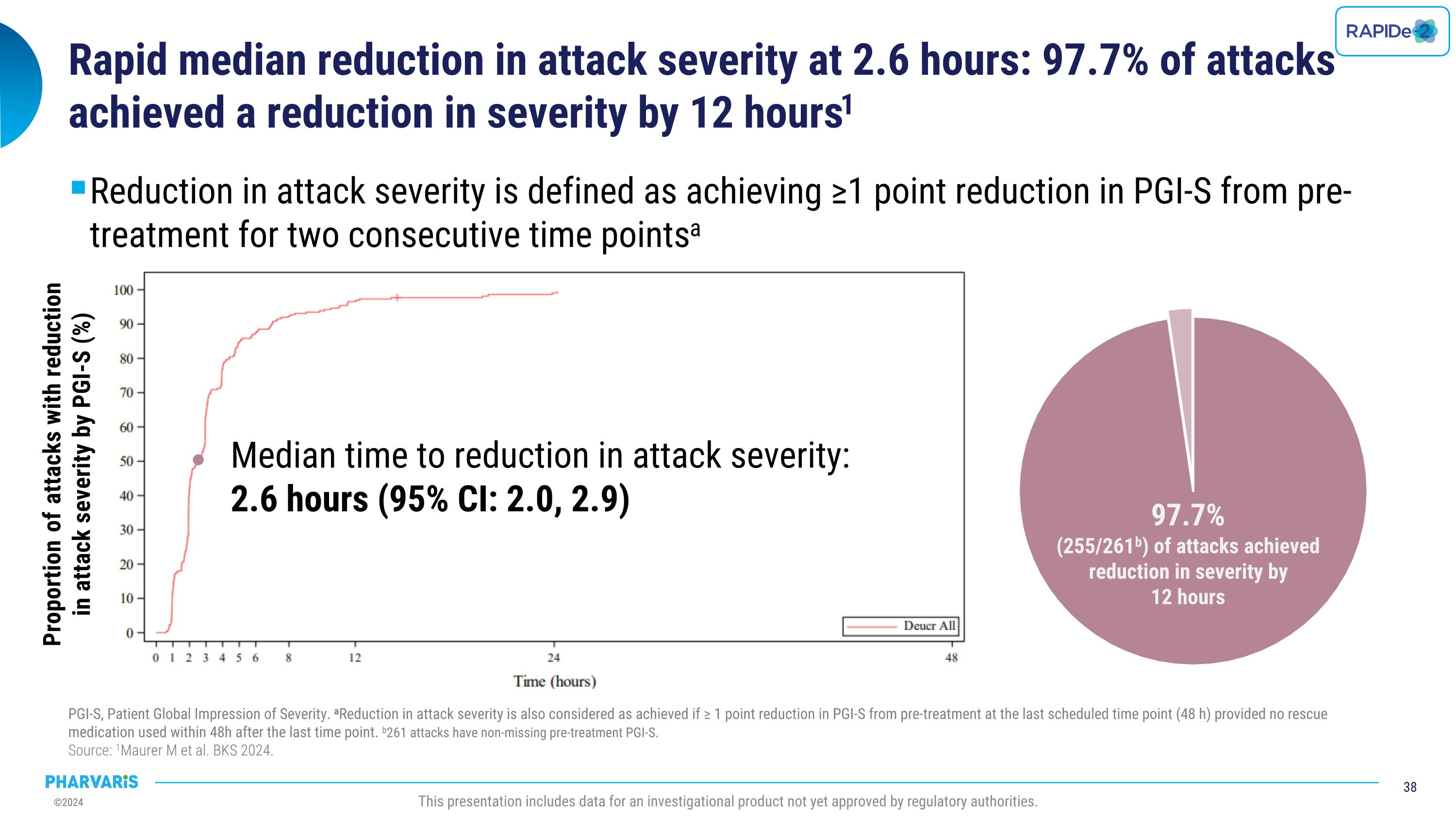
Rapid median reduction in attack severity at 2.6 hours: 97.7% of attacks achieved a reduction in severity by 12 hours1 Reduction in attack severity is defined as achieving ≥1 point reduction in PGI-S from pre-treatment for two consecutive time pointsa PGI-S, Patient Global Impression of Severity. aReduction in attack severity is also considered as achieved if ≥ 1 point reduction in PGI-S from pre-treatment at the last scheduled time point (48 h) provided no rescue medication used within 48h after the last time point. b261 attacks have non-missing pre-treatment PGI-S. �Source: 1Maurer M et al. BKS 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. Median time to reduction in attack severity: �2.6 hours (95% CI: 2.0, 2.9) Proportion of attacks with reduction in attack severity by PGI-S (%)
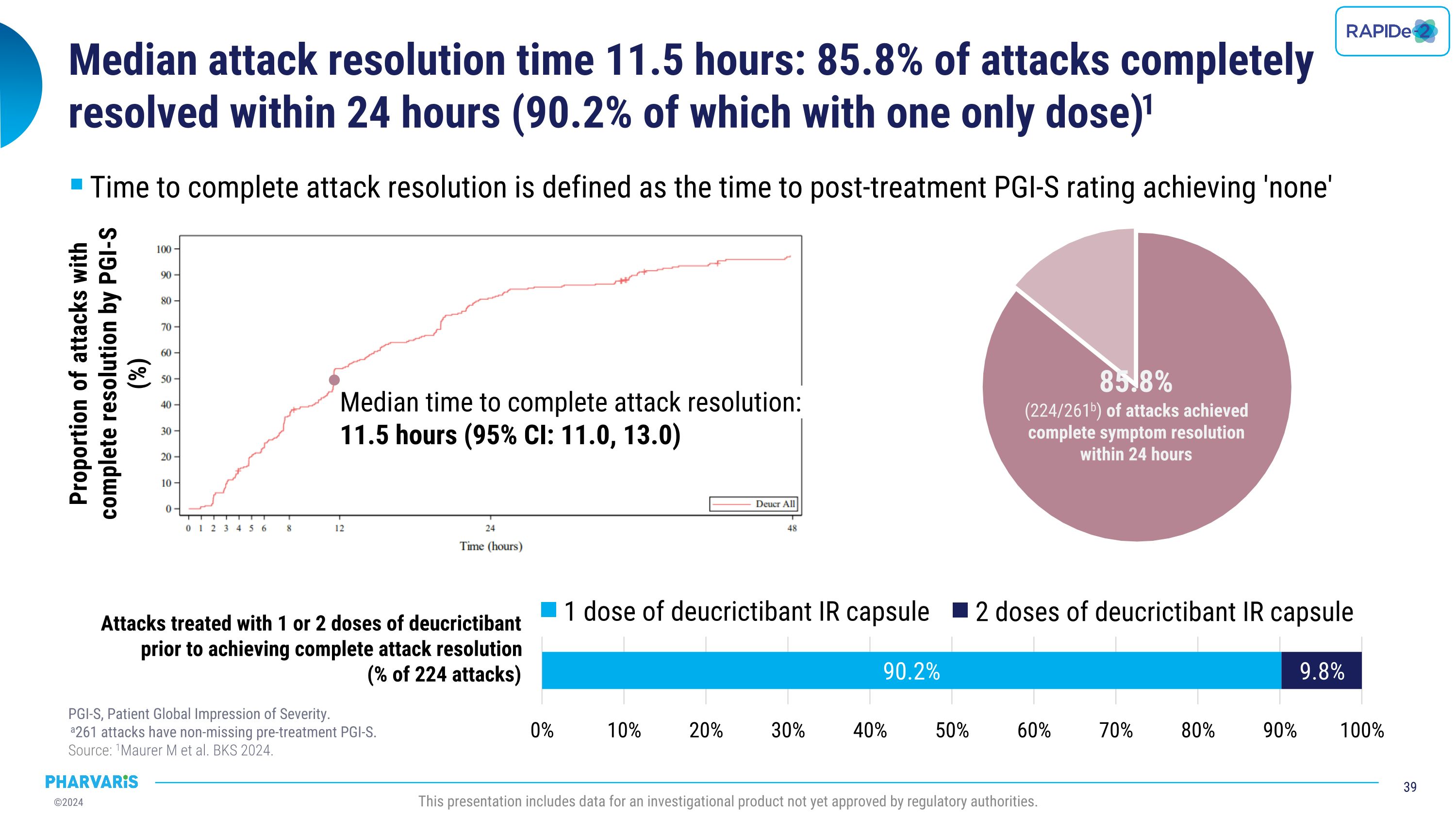
Median attack resolution time 11.5 hours: 85.8% of attacks completely resolved within 24 hours (90.2% of which with one only dose)1 Time to complete attack resolution is defined as the time to post-treatment PGI-S rating achieving 'none' PGI-S, Patient Global Impression of Severity.� a261 attacks have non-missing pre-treatment PGI-S. �Source: 1Maurer M et al. BKS 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. Median time to complete attack resolution: 11.5 hours (95% CI: 11.0, 13.0) Proportion of attacks with complete resolution by PGI-S (%) 1 dose of deucrictibant IR capsule 2 doses of deucrictibant IR capsule Attacks treated with 1 or 2 doses of deucrictibant�prior to achieving complete attack resolution (% of 224 attacks)
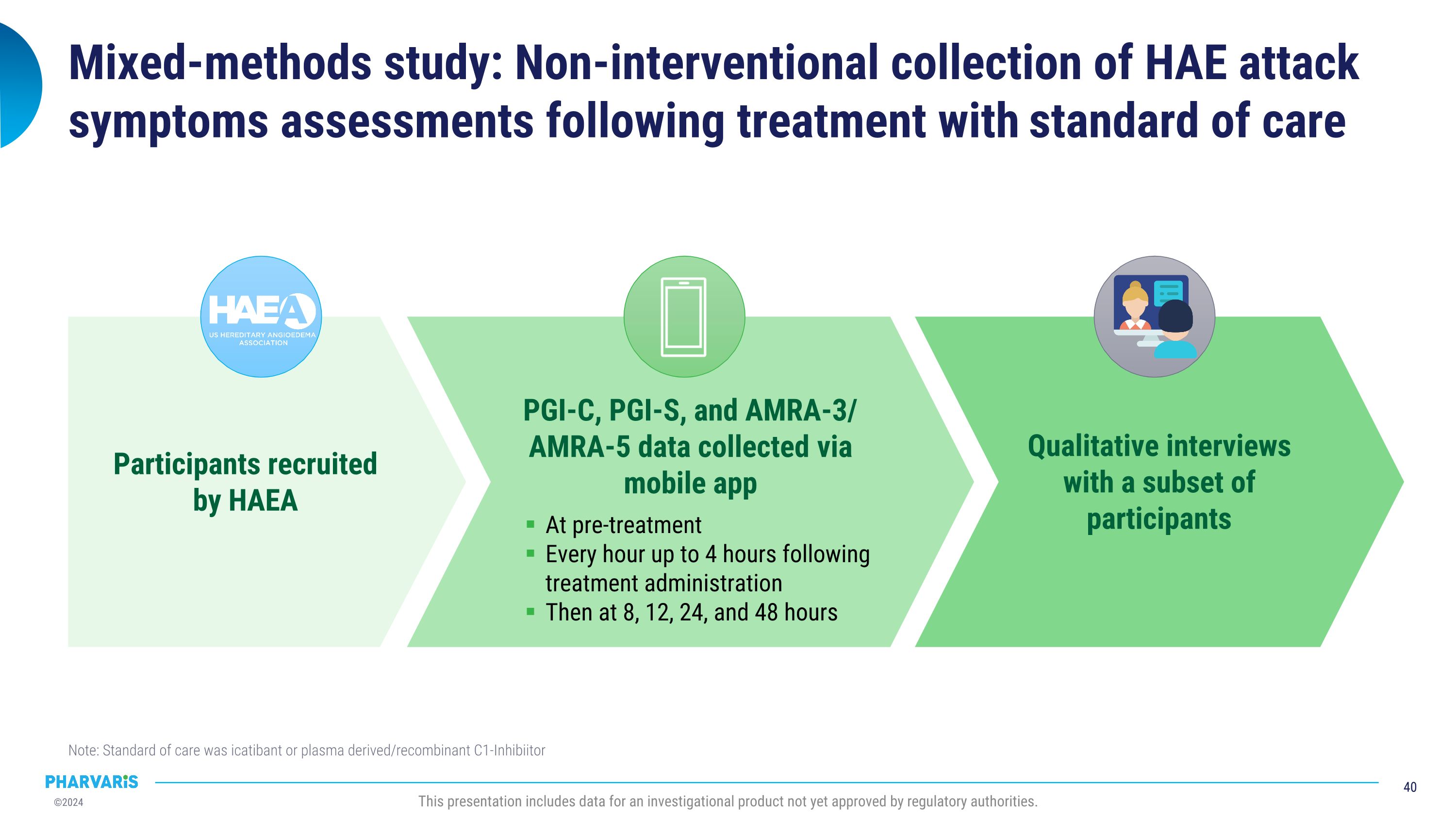
Mixed-methods study: Non-interventional collection of HAE attack symptoms assessments following treatment with standard of care Note: Standard of care was icatibant or plasma derived/recombinant C1-Inhibiitor This presentation includes data for an investigational product not yet approved by regulatory authorities. Participants recruited�by HAEA PGI-C, PGI-S, and AMRA-3/ AMRA-5 data collected via mobile app At pre-treatment Every hour up to 4 hours following treatment administration Then at 8, 12, 24, and 48 hours Qualitative interviews with a subset of participants
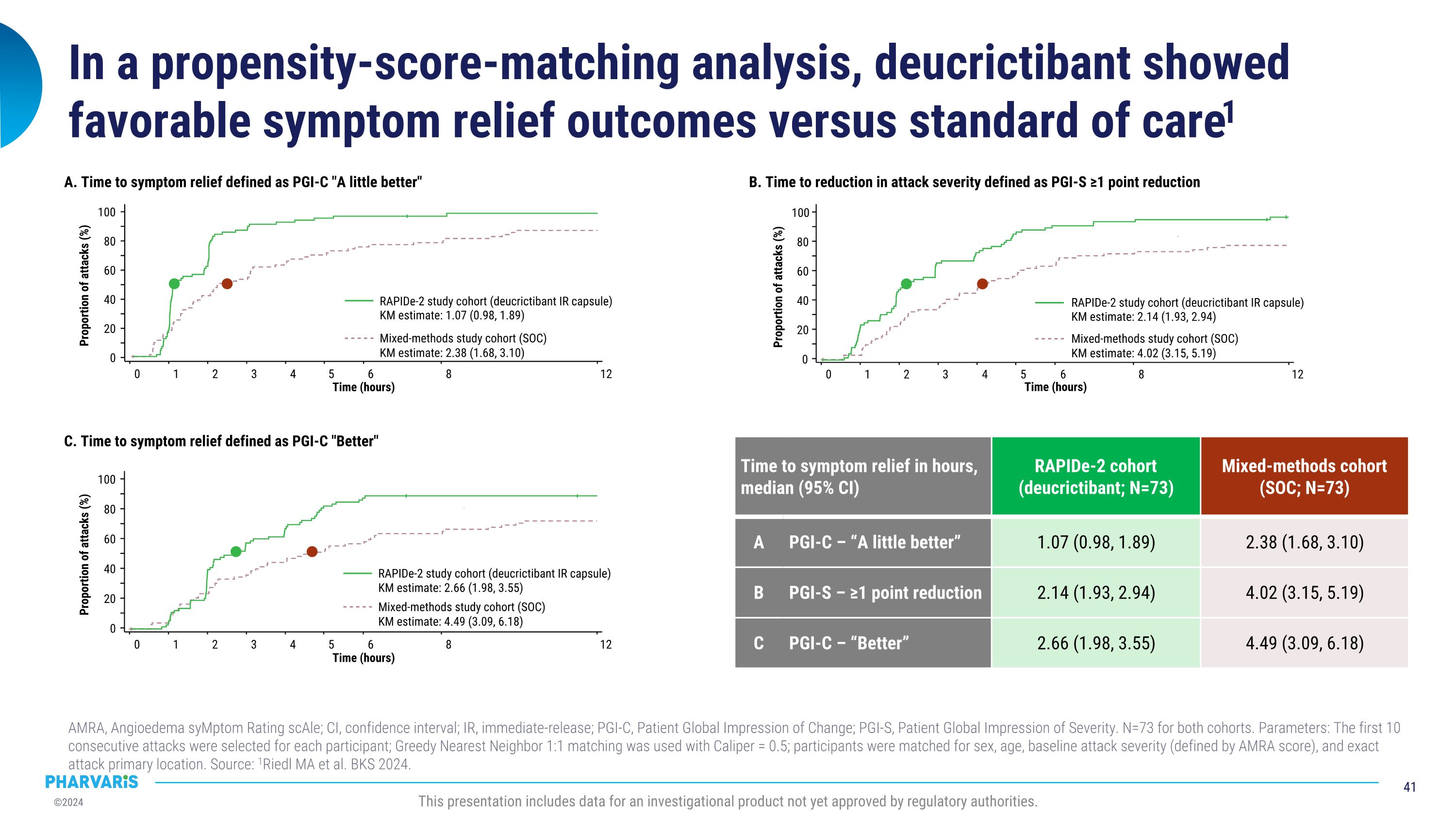
In a propensity-score-matching analysis, deucrictibant showed favorable symptom relief outcomes versus standard of care1 This presentation includes data for an investigational product not yet approved by regulatory authorities. AMRA, Angioedema syMptom Rating scAle; CI, confidence interval; IR, immediate-release; PGI-C, Patient Global Impression of Change; PGI-S, Patient Global Impression of Severity. N=73 for both cohorts. Parameters: The first 10 consecutive attacks were selected for each participant; Greedy Nearest Neighbor 1:1 matching was used with Caliper = 0.5; participants were matched for sex, age, baseline attack severity (defined by AMRA score), and exact attack primary location. Source: 1Riedl MA et al. BKS 2024. A. Time to symptom relief defined as PGI-C "A little better" 0 1 2 3 4 6 12 Time (hours) 0 20 40 60 80 100 Proportion of attacks (%) Mixed-methods study cohort (SOC) KM estimate: 2.38 (1.68, 3.10) RAPIDe-2 study cohort (deucrictibant IR capsule) KM estimate: 1.07 (0.98, 1.89) 5 8 C. Time to symptom relief defined as PGI-C "Better" Proportion of attacks (%) 0 8 1 2 5 3 4 6 12 Time (hours) 0 20 40 60 80 100 Mixed-methods study cohort (SOC) KM estimate: 4.49 (3.09, 6.18) RAPIDe-2 study cohort (deucrictibant IR capsule) KM estimate: 2.66 (1.98, 3.55) B. Time to reduction in attack severity defined as PGI-S ≥1 point reduction Proportion of attacks (%) 0 1 2 5 3 4 6 12 Time (hours) 0 20 40 60 80 100 Mixed-methods study cohort (SOC) KM estimate: 4.02 (3.15, 5.19) RAPIDe-2 study cohort (deucrictibant IR capsule) KM estimate: 2.14 (1.93, 2.94) 8 Time to symptom relief in hours, median (95% CI) RAPIDe-2 cohort (deucrictibant; N=73) Mixed-methods cohort (SOC; N=73) A PGI-C – “A little better” 1.07 (0.98, 1.89) 2.38 (1.68, 3.10) B PGI-S – ≥1 point reduction 2.14 (1.93, 2.94) 4.02 (3.15, 5.19) C PGI-C – “Better” 2.66 (1.98, 3.55) 4.49 (3.09, 6.18)

RAPIDe-3 Clinical Study
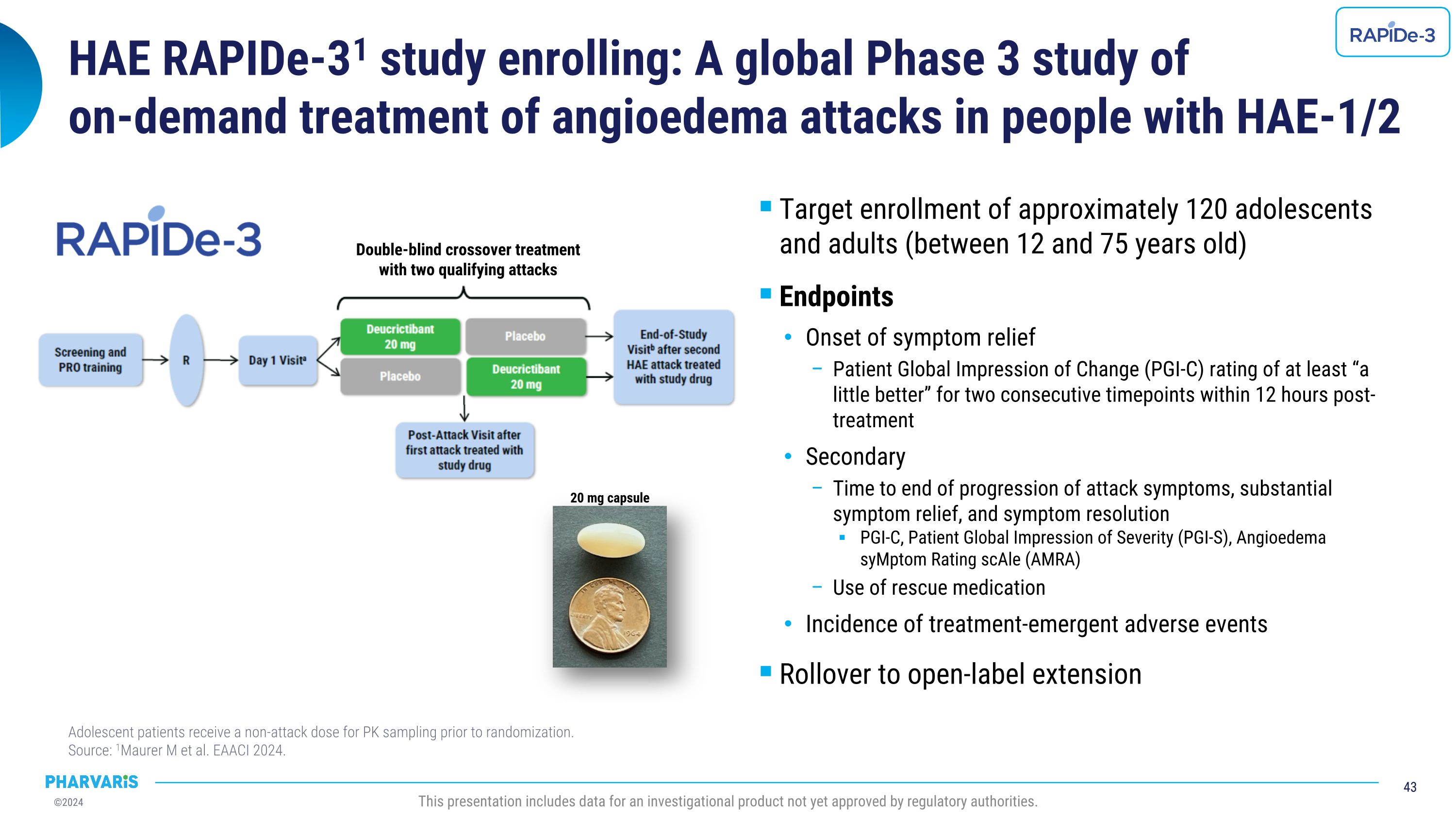
HAE RAPIDe-31 study enrolling: A global Phase 3 study of �on-demand treatment of angioedema attacks in people with HAE-1/2 Target enrollment of approximately 120 adolescents and adults (between 12 and 75 years old) Endpoints Onset of symptom relief Patient Global Impression of Change (PGI-C) rating of at least “a little better” for two consecutive timepoints within 12 hours post-treatment Secondary Time to end of progression of attack symptoms, substantial symptom relief, and symptom resolution PGI-C, Patient Global Impression of Severity (PGI-S), Angioedema syMptom Rating scAle (AMRA) Use of rescue medication Incidence of treatment-emergent adverse events Rollover to open-label extension Adolescent patients receive a non-attack dose for PK sampling prior to randomization.�Source: 1Maurer M et al. EAACI 2024. 20 mg capsule This presentation includes data for an investigational product not yet approved by regulatory authorities. Double-blind crossover treatment with two qualifying attacks
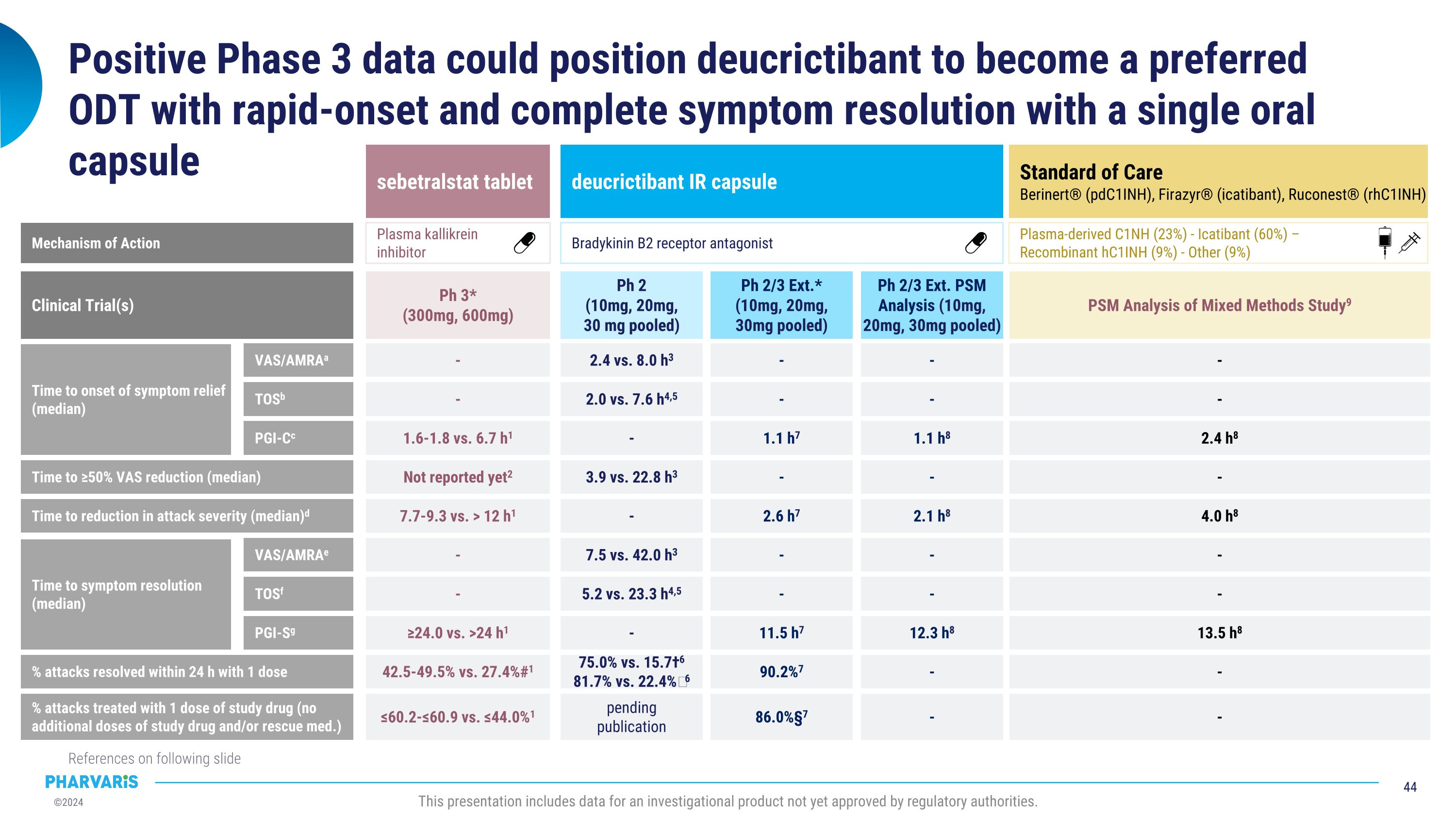
Plasma-derived C1NH (23%) - Icatibant (60%) – �Recombinant hC1INH (9%) - Other (9%) Positive Phase 3 data could position deucrictibant to become a preferred ODT with rapid-onset and complete symptom resolution with a single oral capsule References on following slide This presentation includes data for an investigational product not yet approved by regulatory authorities. Time to onset of symptom relief (median) Standard of Care�Berinert® (pdC1INH), Firazyr® (icatibant), Ruconest® (rhC1INH) - - 2.4 h8 - 4.0 h8 - - 13.5 h8 - PSM Analysis of Mixed Methods Study9 Clinical Trial(s) Time to ≥50% VAS reduction (median) Time to reduction in attack severity (median)d Time to symptom resolution (median) % attacks resolved within 24 h with 1 dose - % attacks treated with 1 dose of study drug (no additional doses of study drug and/or rescue med.) VAS/AMRAa TOSb PGI-Cc VAS/AMRAe TOSf PGI-Sg Mechanism of Action sebetralstat tablet deucrictibant IR capsule 2.4 vs. 8.0 h3 - 2.0 vs. 7.6 h4,5 - - 1.1 h7 3.9 vs. 22.8 h3 - - 2.6 h7 7.5 vs. 42.0 h3 - 5.2 vs. 23.3 h4,5 - - 11.5 h7 75.0% vs. 15.7†6 81.7% vs. 22.4%‖6 90.2%7 - - 1.6-1.8 vs. 6.7 h1 Not reported yet2 7.7-9.3 vs. > 12 h1 - - ≥24.0 vs. >24 h1 42.5-49.5% vs. 27.4%#1 Ph 2 (10mg, 20mg, 30 mg pooled) Ph 2/3 Ext.* (10mg, 20mg, 30mg pooled) Ph 3* (300mg, 600mg) pending�publication 86.0%§7 ≤60.2-≤60.9 vs. ≤44.0%1 Bradykinin B2 receptor antagonist Plasma kallikrein�inhibitor - - 1.1 h8 - 2.1 h8 - - 12.3 h8 - Ph 2/3 Ext. PSM Analysis (10mg, 20mg, 30mg pooled) -
ODT comparison data references * Non-laryngeal and laryngeal attacks included for treatment with study drug. # Symptom resolution assessed by PGI-S. † Symptom resolution assessed by VAS/AMRA. ‖ Symptom resolution assessed by TOS. a. Time to onset of symptom relief by VAS/AMRA defined as ‘VAS-3 ≥30% reduction from pre-treatment score’ in 3. b. Time to onset of symptom relief by TOS defined as ‘The time point when TOS PRO first reaches at least “A little better” for all symptom complexes affected at baseline, ’and no new symptom in any other symptom complex is reported. Relief is confirmed if the improvement is sustained at 2 consecutive time points’ in 4,5. c. Time to beginning (or onset) of symptom relief by PGI-C defined as ‘beginning of symptom relief as assessed in a time-to-event analysis. The beginning of symptom relief was defined as a rating of “a little better” on the 7-point Patient Global Impression of Change (PGI-C) scale (ratings range from “much better” to “much worse”) at two or more consecutive time points within 12 hours after the first administration of the trial agent’ in 1 and as ‘Patient Global Impression of Change (PGI-C) rating of at least “a little better” for 2 consecutive timepoints by 12 hours post-treatment’ in 7,8. d. Time to reduction in attack severity defined as ‘reduction in the severity of the attack, defined as an improved rating on the 5-point Patient Global Impression of Severity (PGI-S) scale (ratings range from “none” to “very severe”) at two or more consecutive time points within 12 hours after the first administration’ in 1 and ‘achieving ≥1 point reduction in the Patient Global Impression of Severity (PGI-S) from pretreatment for 2 consecutive timepoints by 12 hours post-treatment’ in 7,8. e. Time to symptom resolution by VAS/AMRA defined as ‘all 3 individual VAS items ≤10’ in 3. f. Time to symptom resolution by TOS defined as ‘The time point when TOS PRO first reaches “A lot better or resolved” for all symptom complexes affected at baseline, and no new symptom in any other symptom complex is reported’ in.4,5 g. Time to symptom resolution by PGI-S defined as ‘achieving PGI-S rating of “none” at 24 hours post-treatment’ in 1 and as ‘achieving PGI-S rating of “none” at 24 hours post-treatment’ in 7,8 . 1. Riedl MA et al. 2024 N Engl J Med. 2. https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-001226-21/DE (accessed 2 September 2024). 3. Maurer M et al. 2023 AAAAI. 4. Riedl MA et al. 2023 C1INH Workshop. 5. RAPIDe-1 Phase 2 Top-line data presentation, https://ir.pharvaris.com/static-files/733117c4-4632-4bd6-a93b-9484c46e063c (accessed 2 September 2024). 6. Li HH et al. 2024 EAC. 7. Maurer M et al. 2023 BKS. 8. Riedl MA et al. 2024 BKS. 9. Mendivil et al. 2023 UCARE This presentation includes data for an investigational product not yet approved by regulatory authorities.
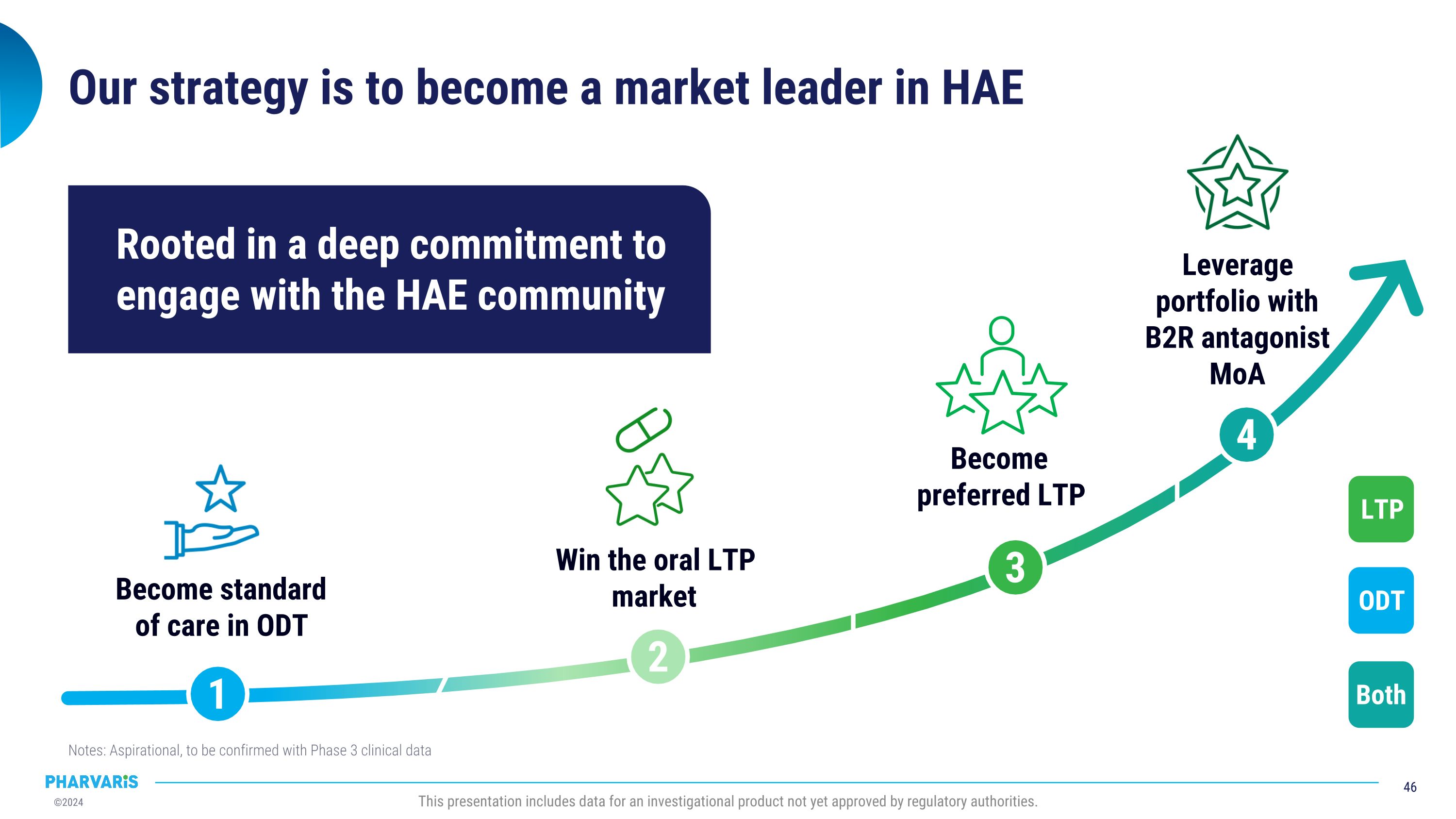
Our strategy is to become a market leader in HAE Notes: Aspirational, to be confirmed with Phase 3 clinical data This presentation includes data for an investigational product not yet approved by regulatory authorities. 1 2 4 3 Win the oral LTP market Become preferred LTP Leverage �portfolio with �B2R antagonist MoA Become standard of care in ODT LTP ODT Both Rooted in a deep commitment to engage with the HAE community

Going Beyond HAE
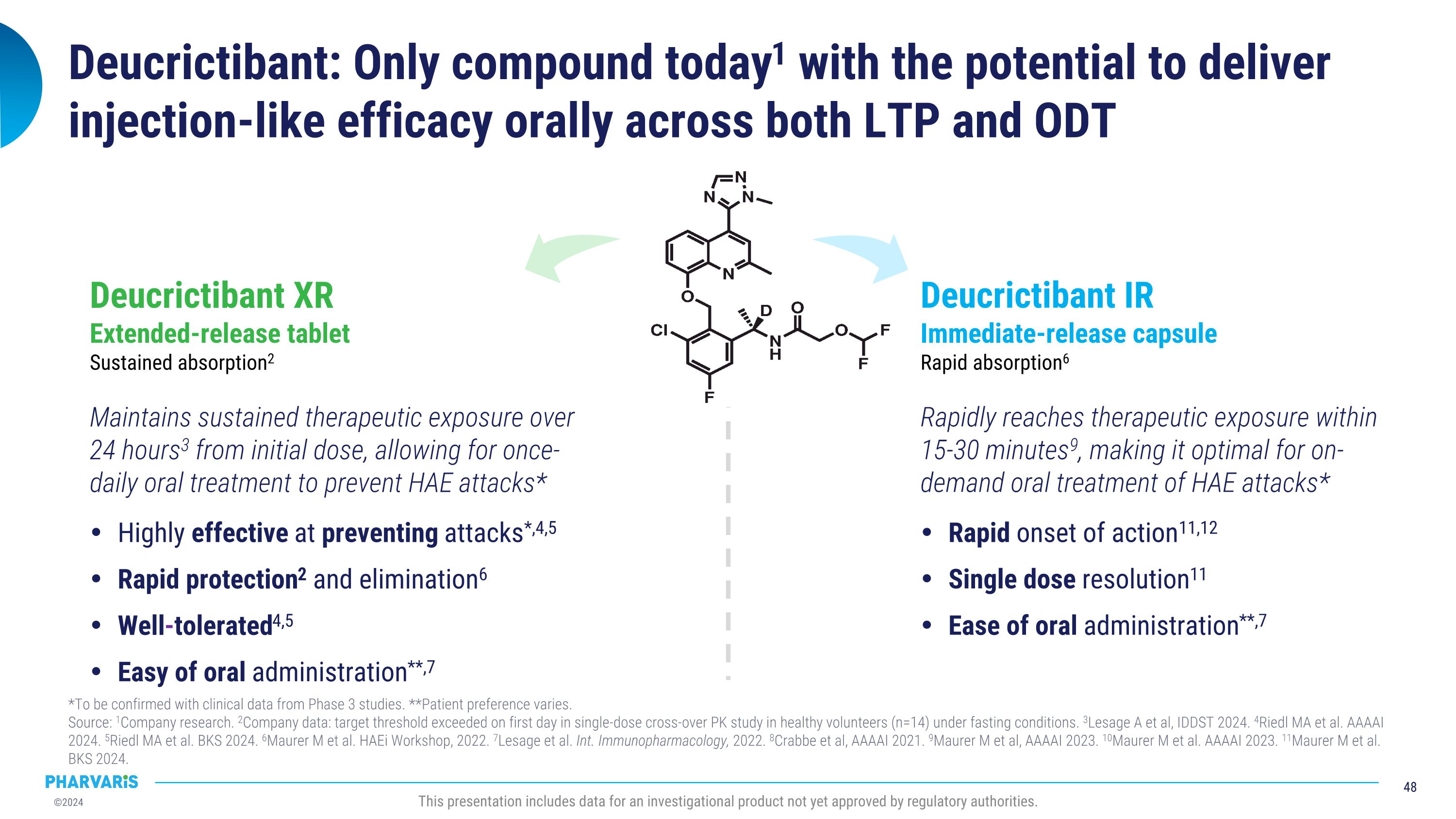
Deucrictibant: Only compound today1 with the potential to deliver injection-like efficacy orally across both LTP and ODT *To be confirmed with clinical data from Phase 3 studies. **Patient preference varies.�Source: 1Company research. 2Company data: target threshold exceeded on first day in single-dose cross-over PK study in healthy volunteers (n=14) under fasting conditions. 3Lesage A et al, IDDST 2024. 4Riedl MA et al. AAAAI 2024. 5Riedl MA et al. BKS 2024. 6Maurer M et al. HAEi Workshop, 2022. 7Lesage et al. Int. Immunopharmacology, 2022. 8Crabbe et al, AAAAI 2021. 9Maurer M et al, AAAAI 2023. 10Maurer M et al. AAAAI 2023. 11Maurer M et al. BKS 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. Deucrictibant XR�Extended-release tablet�Sustained absorption2 Maintains sustained therapeutic exposure over 24 hours3 from initial dose, allowing for once-daily oral treatment to prevent HAE attacks* Highly effective at preventing attacks*,4,5 Rapid protection2 and elimination6 Well-tolerated4,5 Easy of oral administration**,7 Deucrictibant IR�Immediate-release capsule�Rapid absorption6 Rapidly reaches therapeutic exposure within �15-30 minutes9, making it optimal for on-demand oral treatment of HAE attacks* Rapid onset of action11,12 Single dose resolution11 Ease of oral administration**,7
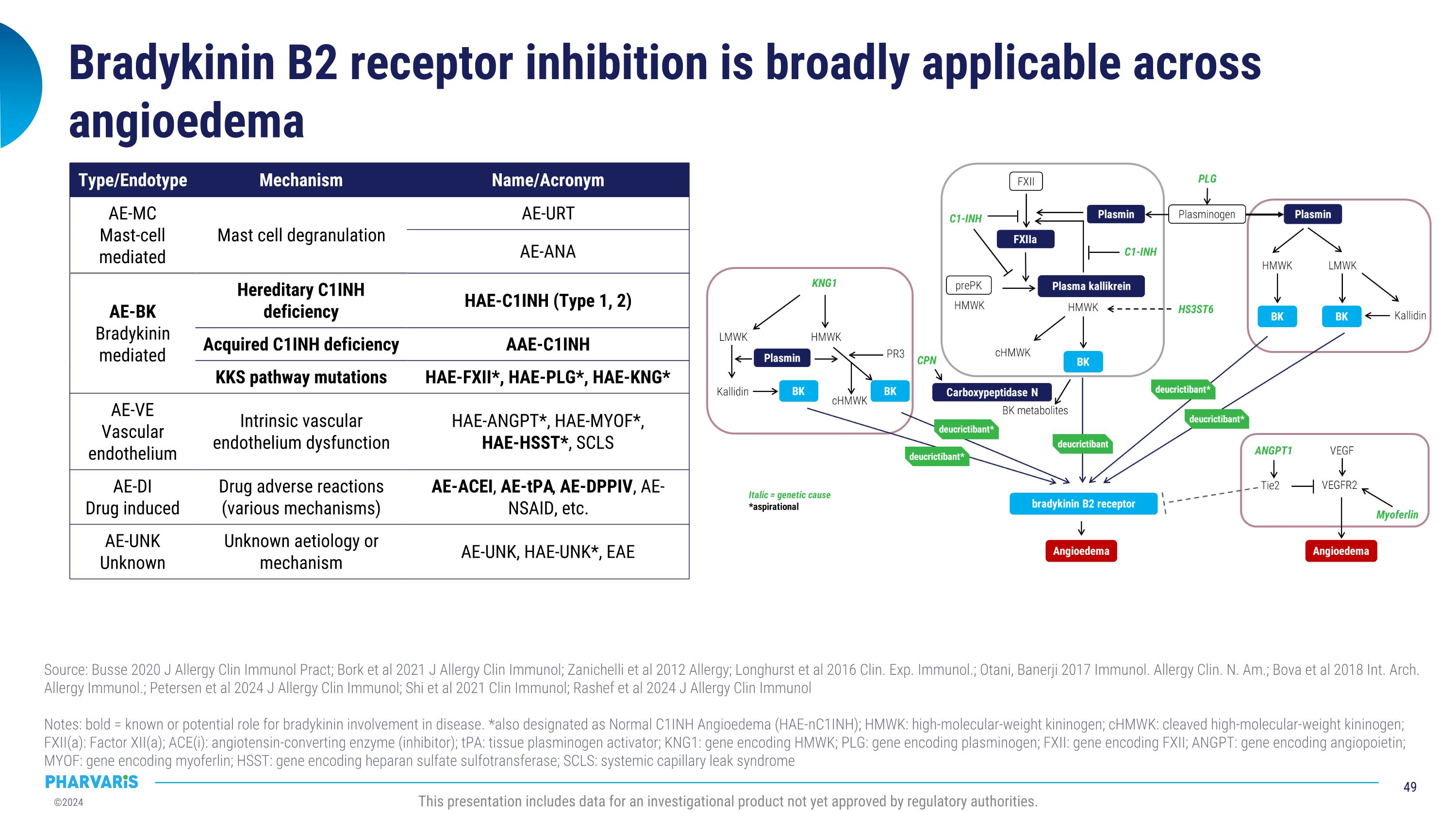
Bradykinin B2 receptor inhibition is broadly applicable across angioedema Source: Busse 2020 J Allergy Clin Immunol Pract; Bork et al 2021 J Allergy Clin Immunol; Zanichelli et al 2012 Allergy; Longhurst et al 2016 Clin. Exp. Immunol.; Otani, Banerji 2017 Immunol. Allergy Clin. N. Am.; Bova et al 2018 Int. Arch. Allergy Immunol.; Petersen et al 2024 J Allergy Clin Immunol; Shi et al 2021 Clin Immunol; Rashef et al 2024 J Allergy Clin Immunol Notes: bold = known or potential role for bradykinin involvement in disease. *also designated as Normal C1INH Angioedema (HAE-nC1INH); HMWK: high-molecular-weight kininogen; cHMWK: cleaved high-molecular-weight kininogen; FXII(a): Factor XII(a); ACE(i): angiotensin-converting enzyme (inhibitor); tPA: tissue plasminogen activator; KNG1: gene encoding HMWK; PLG: gene encoding plasminogen; FXII: gene encoding FXII; ANGPT: gene encoding angiopoietin; MYOF: gene encoding myoferlin; HSST: gene encoding heparan sulfate sulfotransferase; SCLS: systemic capillary leak syndrome Type/Endotype Mechanism Name/Acronym AE-MC Mast-cell mediated Mast cell degranulation AE-URT AE-ANA AE-BK Bradykinin mediated Hereditary C1INH deficiency HAE-C1INH (Type 1, 2) Acquired C1INH deficiency AAE-C1INH KKS pathway mutations HAE-FXII*, HAE-PLG*, HAE-KNG* AE-VE Vascular endothelium Intrinsic vascular �endothelium dysfunction HAE-ANGPT*, HAE-MYOF*, �HAE-HSST*, SCLS AE-DI Drug induced Drug adverse reactions �(various mechanisms) AE-ACEI, AE-tPA, AE-DPPIV, AE-NSAID, etc. AE-UNK Unknown Unknown aetiology or mechanism AE-UNK, HAE-UNK*, EAE This presentation includes data for an investigational product not yet approved by regulatory authorities.
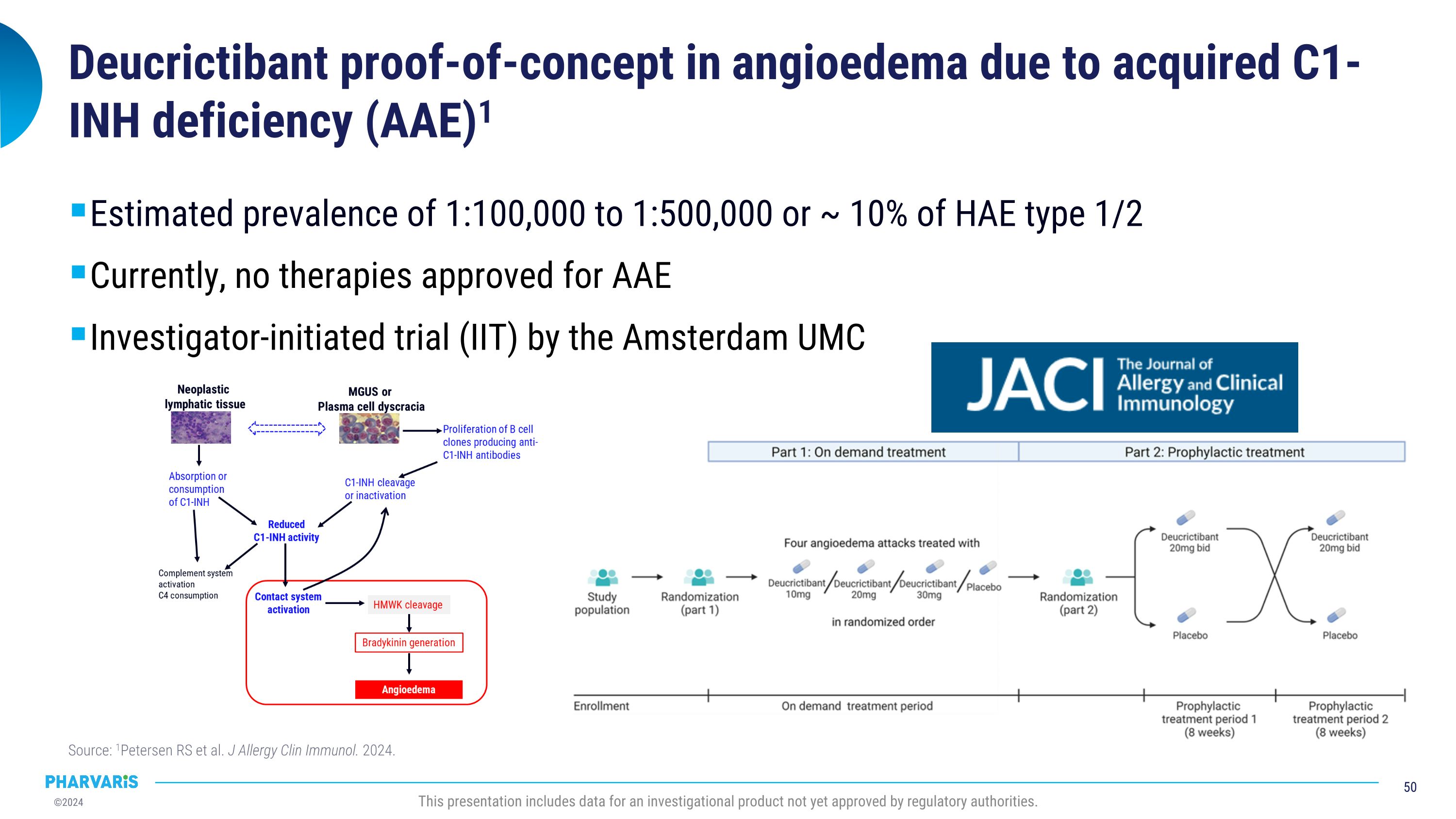
Deucrictibant proof-of-concept in angioedema due to acquired C1-INH deficiency (AAE)1 Estimated prevalence of 1:100,000 to 1:500,000 or ~ 10% of HAE type 1/2 Currently, no therapies approved for AAE Investigator-initiated trial (IIT) by the Amsterdam UMC Source: 1Petersen RS et al. J Allergy Clin Immunol. 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities.
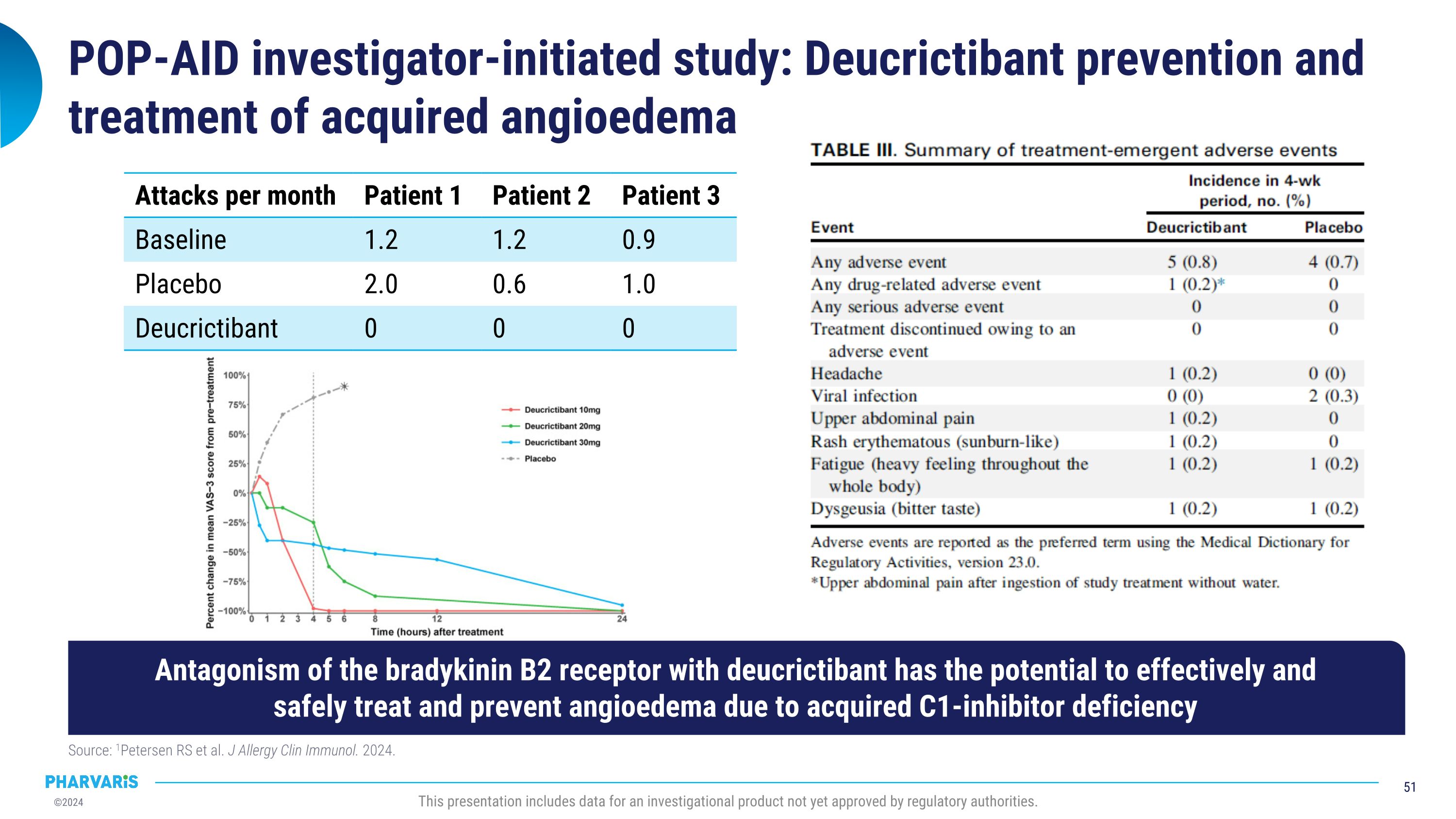
POP-AID investigator-initiated study: Deucrictibant prevention and treatment of acquired angioedema Source: 1Petersen RS et al. J Allergy Clin Immunol. 2024. This presentation includes data for an investigational product not yet approved by regulatory authorities. Attacks per month Patient 1 Patient 2 Patient 3 Baseline 1.2 1.2 0.9 Placebo 2.0 0.6 1.0 Deucrictibant 0 0 0 Antagonism of the bradykinin B2 receptor with deucrictibant has the potential to effectively and�safely treat and prevent angioedema due to acquired C1-inhibitor deficiency
Rare allergy & immunology conditions (commercial) BK-mediated diseases (R&D) BK-mediated angioedema: nC1, AAE Pharvaris aspires to leverage its foundational B2R expertise to develop therapies for conditions beyond HAE : HAE Type 1/2 This presentation includes data for an investigational product not yet approved by regulatory authorities.

www.pharvaris.com NASDAQ: PHVS This presentation includes data for an investigational product not yet approved by regulatory authorities.
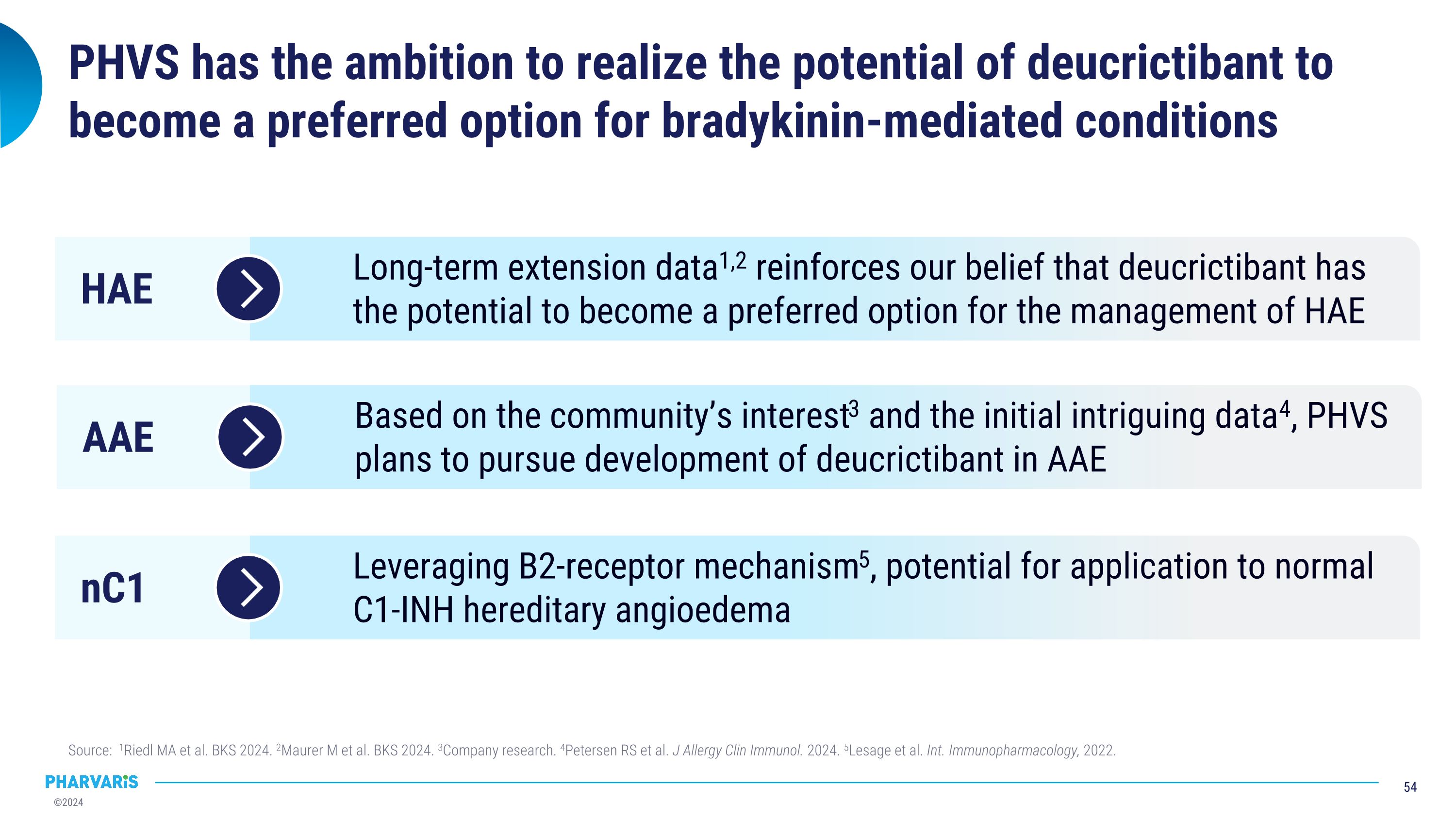
PHVS has the ambition to realize the potential of deucrictibant to become a preferred option for bradykinin-mediated conditions Source: 1Riedl MA et al. BKS 2024. 2Maurer M et al. BKS 2024. 3Company research. 4Petersen RS et al. J Allergy Clin Immunol. 2024. 5Lesage et al. Int. Immunopharmacology, 2022. HAE Long-term extension data1,2 reinforces our belief that deucrictibant has the potential to become a preferred option for the management of HAE AAE Based on the community’s interest3 and the initial intriguing data4, PHVS plans to pursue development of deucrictibant in AAE nC1 Leveraging B2-receptor mechanism5, potential for application to normal C1-INH hereditary angioedema
Grafico Azioni Pharvaris NV (NASDAQ:PHVS)
Storico
Da Nov 2024 a Dic 2024

Grafico Azioni Pharvaris NV (NASDAQ:PHVS)
Storico
Da Dic 2023 a Dic 2024
