0001818331false00018183312024-01-082024-01-080001818331us-gaap:CommonClassAMember2024-01-082024-01-080001818331us-gaap:WarrantMember2024-01-082024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (date of earliest event reported): January 8, 2024
Commission file number 001-39482
GeneDx Holdings Corp.
(Exact name of registrant as specified in its charter)
| | | | | |
Delaware | 85-1966622 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| |
333 Ludlow Street, North Tower; 6th Floor Stamford, Connecticut 06902 |
| (Address of Principal Executive Offices) (Zip Code) |
Registrant's telephone number, including area code: (800) 298-6470
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol | | Name of each exchange on which registered |
| Class A common stock, par value $0.0001 per share | | WGS | | The Nasdaq Stock Market LLC |
| Warrants to purchase one share of Class A common stock, each at an exercise price of $379.50 per share | | WGSWW | | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
The information set forth in Item 7.01 below is incorporated by reference into this Item 2.02.
Item 7.01 Regulation FD Disclosure.
On January 8, 2024, GeneDx Holdings Corp. (the “Company”) issued a press release (the “Press Release”) announcing the Company’s expectations regarding its preliminary, unaudited revenue for the fourth quarter and the year ended 2023, exome and genome test result volumes for the fourth quarter and cash, cash equivalents, marketable securities and restricted cash as of December 31, 2023. A copy of the Press Release is included with this Form 8-K for convenience and attached hereto as Exhibit 99.1. Also on January 8, 2024, the Company is furnishing as Exhibit 99.2 hereto a copy of the investor presentation to be used at the 42nd Annual J.P. Morgan Healthcare Conference event.
The information furnished under Items 2.02 and 7.01 of this Current Report on Form 8-K, including Exhibit 99.1 hereto, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits
(d) Exhibits
| | | | | |
Exhibit No | Description |
| 99.1 | |
| 99.2 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | |
| | GeneDx Holdings Corp. |
| | |
| Date: | January 8, 2024 | By: | /s/ Katherine Stueland |
| | Name: | Katherine Stueland |
| | Title: | Chief Executive Officer |
GeneDx Announces Preliminary 2023 Financial Results
Expects to exceed revised 2023 revenue guidance with fourth quarter 2023 revenue from continuing operations1 of more than $57M with at least 63% year-over-year revenue growth for exome and genome test revenue
Reduced fourth quarter 2023 cash burn 51% year-over-year and 21% sequentially
Ending December 31, 2023 cash position of at least $131 million
Reiterating the path to profitability in 2025
STAMFORD, Conn, Jan. 08, 2024 -- GeneDx Holdings Corp. (Nasdaq: WGS), a leader in delivering improved health outcomes through genomic and clinical insights, today reported preliminary financial results for the fourth quarter and full year of 2023.
"We had an incredibly strong fourth quarter as we continued to drive utilization of our industry-leading exome and genome products, improve our average reimbursement rate, and meaningfully reduce our cash burn,” said Katherine Stueland, President and Chief Executive Officer of GeneDx. “With continued strong execution across these metrics in 2024, we’re confident in our ability to reach profitability in 2025, ultimately keeping our eye on the goal of diagnosing inherited disease earlier, connecting people with the right treatment, and driving a healthier future for as many families as possible.”
Preliminary 2023 Financial Results (Unaudited)
Continuing Operations Results: 1
Management expects GeneDx to report:
•revenues of at least $193 million for full year 2023
•revenues of at least $57 million in the fourth quarter, an increase of 24% year-over-year and 13% sequentially;
•exome and genome test revenues of at least $38 million in the fourth quarter, an increase of 63% year-over-year and 12% sequentially; and
•15,663 exome and genome test results in the fourth quarter, an increase of 99% year-over-year and 19% sequentially with exome and genome representing 27% of all tests result volume in the fourth quarter.
Total Company Results: 1
Management expects GeneDx to report:
·revenues of at least $203 million for full year 2023;
·revenues of at least $58 million in the fourth quarter; and
•Cash, cash equivalents, marketable securities and restricted cash of $131 million as of December 31, 2023. Total Company use of cash for the fourth quarter of 2023 was $33 million, an improvement of 51% year-over-year and 21% sequentially. The use of cash in the fourth quarter included $5 million in scheduled payments under the Legacy Sema4
payer settlement entered into in 2022, $3 million to discharge operating payables for the exited reproductive health business and $1 million in severance payments related to the previously announced cost reduction initiative. Excluding these items, representative continuing operations cash burn was $24 million in the fourth quarter of 2023.
"Our fourth quarter preliminary results, which exceeded previously issued guidance, provide great momentum heading into 2024. Growth is strong and losses are consistently narrowing with each passing quarter. We can confidently reiterate the anticipated path to profitability in 2025,” said Kevin Feeley, Chief Financial Officer of GeneDx.
GeneDx has not completed the preparation of its consolidated financial statements for the year ended December 31, 2023. The preliminary, unaudited results presented in this press release for the year ended December 31, 2023, are based on current expectations and are subject to adjustment, as the company completes the preparation of its 2023 year-end consolidated financial statements and its 2023 year-end audit.
GeneDx will release financial results for the fourth quarter and full year of 2023 after the market closes on Tuesday, February 20, 2024. On the same day, Katherine Stueland, President and Chief Executive Officer of GeneDx, and Kevin Feeley, Chief Financial Officer of GeneDx, will host a conference call to discuss financial and operating results at 4:30 p.m. Eastern Time.
Investors interested in listening to the conference call are required to register online. A live and archived webcast of the event will be available on the “Events” section of the GeneDx investor relations website at https://ir.genedx.com/.
1 Preliminary results from continuing operations exclude the results of the discontinued Legacy Sema4 diagnostic testing business. Preliminary total company results include GeneDx’s continuing operations and the financial impacts of exited Legacy Sema4 business activities.
Safe Harbor Statements
This press release contains certain forward-looking statements within the meaning of the federal securities laws, including statements regarding our future performance and our market opportunity, including our preliminary, unaudited fourth quarter and full year 2023 revenue, fourth quarter and full year 2023 test result volumes, December 31, 2023 cash balance and use of cash for the fourth quarter of 2023, our expectation of turning profitable in 2025 and our expectations for our growth and future investment in our business. These forward-looking statements generally are identified by the words “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Many factors could cause actual future events to differ materially from the
forward-looking statements in this press release, including but not limited to: (i) our ability to implement business plans, goals and forecasts, and identify and realize additional opportunities, (ii) the risk of downturns and a changing regulatory landscape in the highly competitive healthcare industry, (iii) the size and growth of the market in which we operate, (iv) our ability to pursue our new strategic direction, and (vi) our ability to enhance our artificial intelligence tools that we use in our clinical interpretation platform. The foregoing list of factors is not exhaustive. You should carefully consider the foregoing factors and the other risks and uncertainties described in the “Risk Factors” section of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the U.S. Securities and Exchange Commission (the “SEC”) on March 16, 2023 and other documents filed by us from time to time with the SEC. These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and we assume no obligation and do not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise. We do not give any assurance that we will achieve our expectations.
About GeneDx
GeneDx, (Nasdaq: WGS) delivers personalized and actionable health insights to inform diagnosis, direct treatment and improve drug discovery. The company is uniquely positioned to accelerate the use of genomic and large-scale clinical information to enable precision medicine as the standard of care. GeneDx is at the forefront of transforming healthcare through its industry-leading exome and genome testing and interpretation, fueled by one of the world’s largest rare disease data sets. For more information, please visit genedx.com and connect with us on LinkedIn, Facebook, and Instagram.
Media contact
Press@GeneDx.com
Investor contact
Investors@genedx.com

One test. Big picture. Brighter futures. 42nd Annual JPMorgan Healthcare Conference January 8, 2024 Katherine Stueland, President and Chief Executive Officer GeneDx (Nasdaq: WGS) Exhibit 99.2

2 Disclaimer This presentation contains forward-looking statements under the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that do not relate to historical facts and events and such statements and opinions pertaining to the future that, for example, contain wording such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward- looking statements contained in this presentation may include, but are not limited to, statements about: our future performance and our market opportunity, our preliminary, unaudited fourth quarter and full year 2023 revenue, fourth quarter and full year 2023 test result volumes, our expectations regarding our gross margin profile in 2024 and beyond, our use of cash for continuing operations and our cash burn in 2023, our expectation of turning profitable in 2025, our expectations for our growth and future investment in our business, our expectations regarding our plans to pursue a new strategic direction, improve our operational efficiency and reduce our cash burn and our ability to scale to profitability, the associated cost savings of our business exits and impact on our gross margins. We cannot assure that the forward-looking statements in this presentation will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. The forward-looking statements and opinions contained in this presentation are based on our management’s beliefs and assumptions and are based upon information currently available to our management as of the date of this presentation and, while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. Many factors could cause actual future events to differ materially from the forward-looking statements in this presentation, including but not limited to: (i) the completion of the preparation of our 2023 year-end financial statements (including all required disclosures) and our 2023 year-end audit, (ii) the ability to implement business plans, goals and forecasts, and identify and realize additional opportunities, (iii) the risk of downturns and a changing regulatory landscape in the highly competitive healthcare industry, (iv) the size and growth of the market in which we operate, and (v) our ability to pursue our new strategic direction. The information, opinions and forward-looking statements contained in this announcement speak only as of its date and are subject to change without notice. This presentation contains estimates, projections and other information concerning our industry, our business, and the markets for our products and services. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from our own internal estimates and research as well as from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. While we believe our internal company research as to such matters is reliable and the market definitions are appropriate, neither such research nor these definitions have been verified by any independent source. We discuss these and other risks and uncertainties in greater detail in the sections entitled “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our periodic reports and other filings we make with the SEC from time to time. Given these uncertainties, you should not place undue reliance on the forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations. We file reports, proxy statements, and other information with the SEC. Such reports, proxy statements, and other information concerning us are available www.sec.gov. Requests for copies of such documents should be directed to our Investor Relations department at GeneDx Holdings Corp. 333 Ludlow Street, North Tower, Stamford, Connecticut, 06902. Our telephone number is 800-298-6470. GeneDx has not completed the preparation of its consolidated financial statements for the year ended December 31, 2023. The preliminary, unaudited results presented in this presentation for the year ended December 31, 2023, are based on current expectations and are subject to adjustment, as the company completes the preparation of its 2023 year-end consolidated financial statements and its 2023 year-end audit.

4Q 2023 preliminary results 3 Expects to exceed revised 2023 revenue guidance with fourth quarter 2023 revenue from continuing operations expected to be least $57M with 63% year-over-year revenue growth for exome and genome test revenue Reiterating the path to profitability in 2025 Reduced cash burn 51% year-over-year and 21% sequentially Ending December 31, 2023 cash, cash equivalents, marketable securities and restricted cash position of $131 million 2 Preliminary results from continuing operations include exclude the results of the discontinued Legacy Sema4 diagnostic testing business. Preliminary total company results include GeneDx's continuing operations and the financial impacts of exited Legacy Sema4 business activities. 2 1 Revised 2023 revenue guidance was $187-192M in revenues from continuing operations. Management expects GeneDx to report full year 2023 revenue from continuing operations of at least $193 million. Total company revenues are expected to be at least $203M and at least $58M for the full year and four quarter 2023, respectively. 1
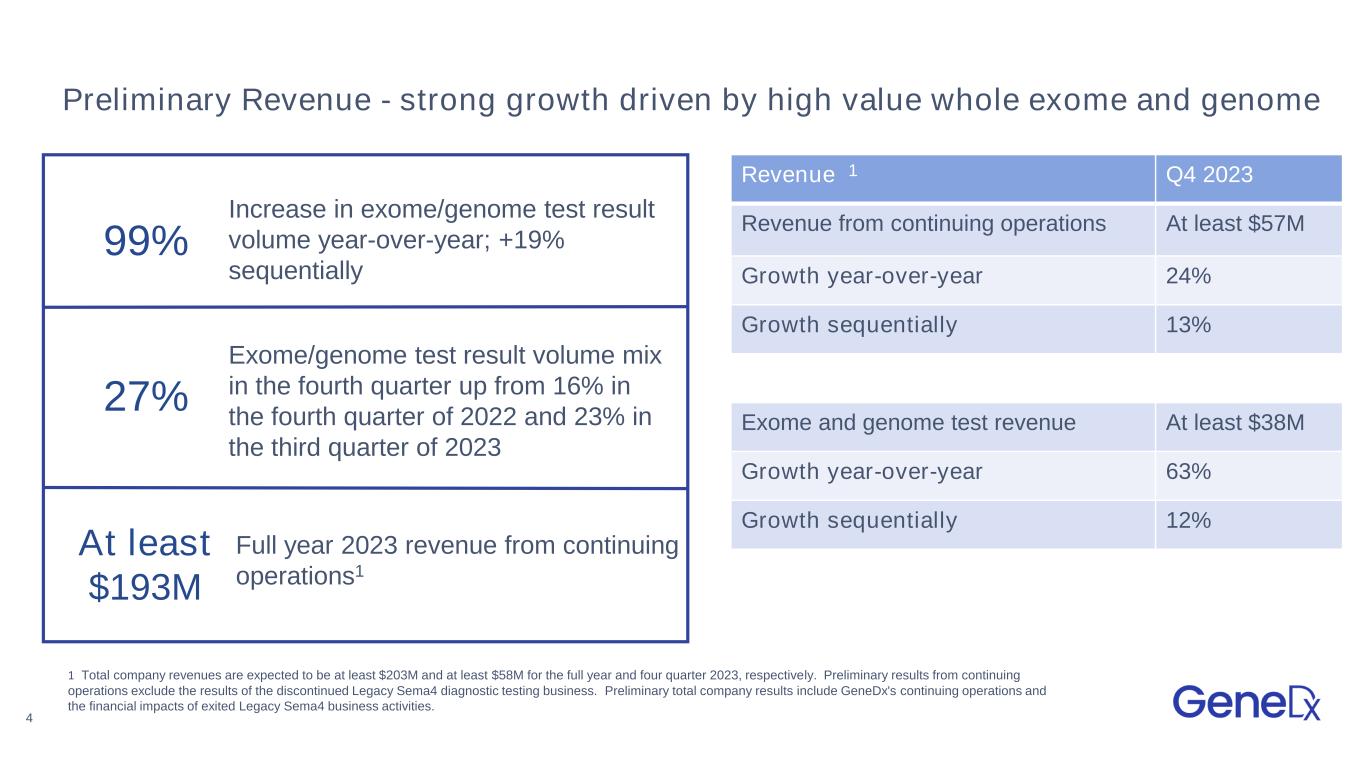
4 Preliminary Revenue - strong growth driven by high value whole exome and genome Revenue Q4 2023 Revenue from continuing operations At least $57M Growth year-over-year 24% Growth sequentially 13% Exome and genome test revenue At least $38M Growth year-over-year 63% Growth sequentially 12% Increase in exome/genome test result volume year-over-year; +19% sequentially 99% 27% Exome/genome test result volume mix in the fourth quarter up from 16% in the fourth quarter of 2022 and 23% in the third quarter of 2023 Full year 2023 revenue from continuing operations1 At least $193M 1 Total company revenues are expected to be at least $203M and at least $58M for the full year and four quarter 2023, respectively. Preliminary results from continuing operations exclude the results of the discontinued Legacy Sema4 diagnostic testing business. Preliminary total company results include GeneDx's continuing operations and the financial impacts of exited Legacy Sema4 business activities. 1
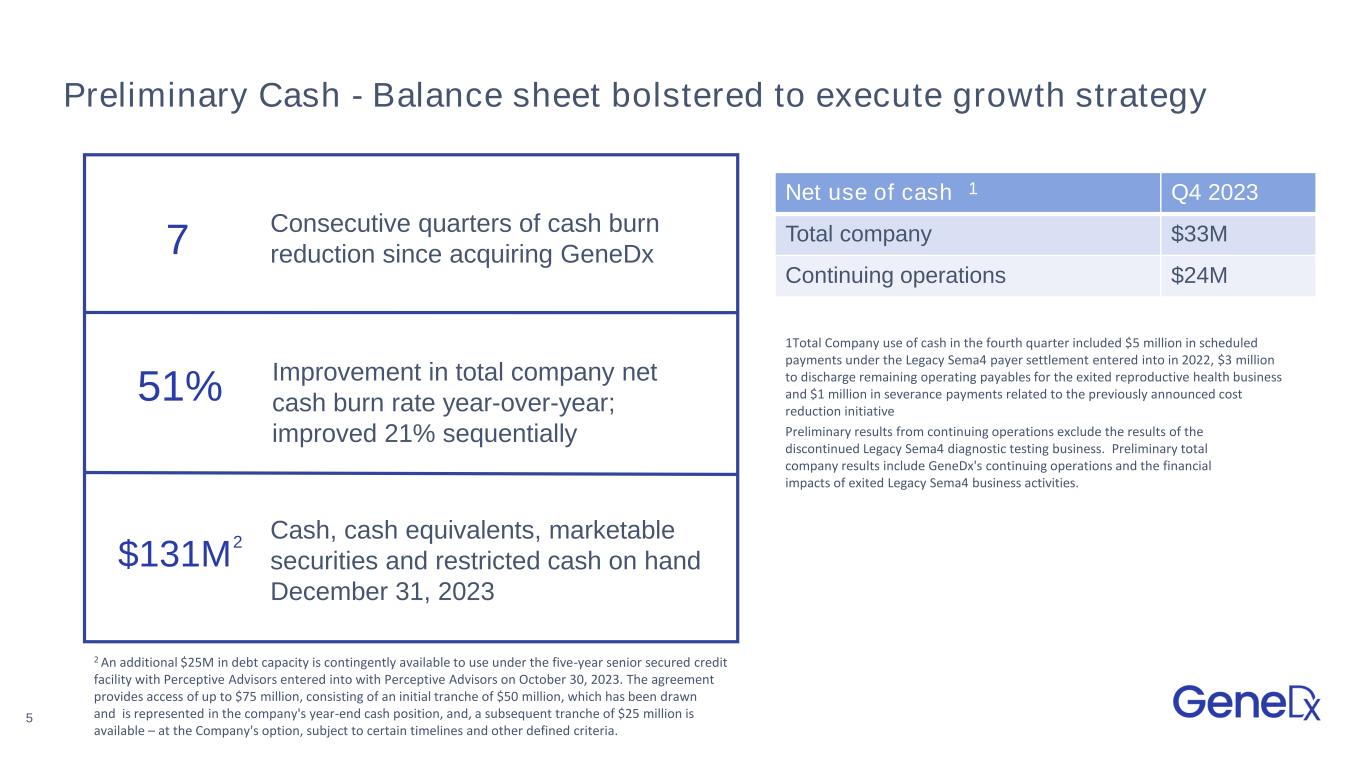
5 Preliminary Cash - Balance sheet bolstered to execute growth strategy Net use of cash Q4 2023 Total company $33M Continuing operations $24M Cash, cash equivalents, marketable securities and restricted cash on hand December 31, 2023 $131M Consecutive quarters of cash burn reduction since acquiring GeneDx7 51% Improvement in total company net cash burn rate year-over-year; improved 21% sequentially 1Total Company use of cash in the fourth quarter included $5 million in scheduled payments under the Legacy Sema4 payer settlement entered into in 2022, $3 million to discharge remaining operating payables for the exited reproductive health business and $1 million in severance payments related to the previously announced cost reduction initiative 2 An additional $25M in debt capacity is contingently available to use under the five-year senior secured credit facility with Perceptive Advisors entered into with Perceptive Advisors on October 30, 2023. The agreement provides access of up to $75 million, consisting of an initial tranche of $50 million, which has been drawn and is represented in the company's year-end cash position, and, a subsequent tranche of $25 million is available – at the Company's option, subject to certain timelines and other defined criteria. 2 1 Preliminary results from continuing operations exclude the results of the discontinued Legacy Sema4 diagnostic testing business. Preliminary total company results include GeneDx's continuing operations and the financial impacts of exited Legacy Sema4 business activities.
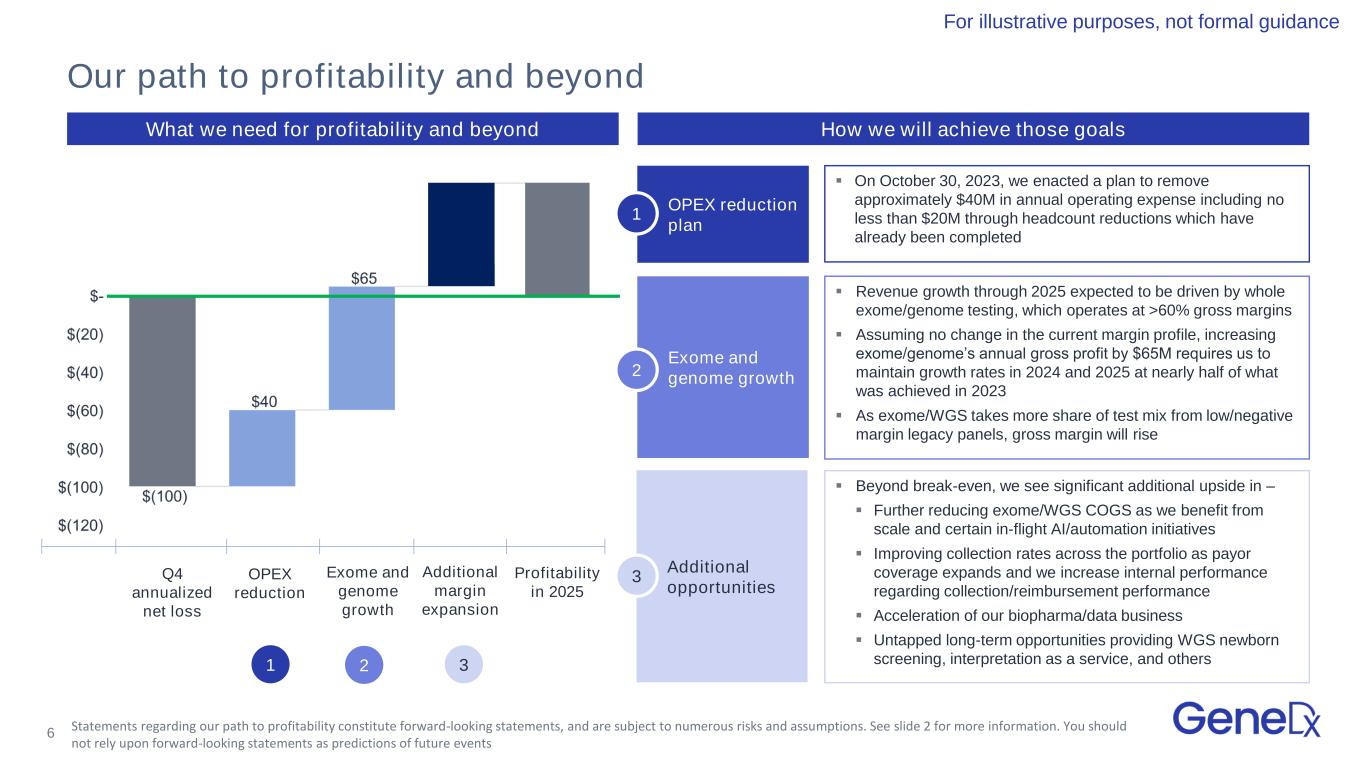
6 Confidential and Proprietary; Do Not Distribute OPEX reduction plan What we need for profitability and beyond ▪ On October 30, 2023, we enacted a plan to remove approximately $40M in annual operating expense including no less than $20M through headcount reductions which have already been completed ▪ Revenue growth through 2025 expected to be driven by whole exome/genome testing, which operates at >60% gross margins ▪ Assuming no change in the current margin profile, increasing exome/genome’s annual gross profit by $65M requires us to maintain growth rates in 2024 and 2025 at nearly half of what was achieved in 2023 ▪ As exome/WGS takes more share of test mix from low/negative margin legacy panels, gross margin will rise ▪ Beyond break-even, we see significant additional upside in – ▪ Further reducing exome/WGS COGS as we benefit from scale and certain in-flight AI/automation initiatives ▪ Improving collection rates across the portfolio as payor coverage expands and we increase internal performance regarding collection/reimbursement performance ▪ Acceleration of our biopharma/data business ▪ Untapped long-term opportunities providing WGS newborn screening, interpretation as a service, and others Exome and genome growth Additional opportunities Q4 annualized net loss OPEX reduction Additional margin expansion Exome and genome growth Profitability in 2025 Our path to profitability and beyond For illustrative purposes, not formal guidance How we will achieve those goals 1 2 3 1 2 3 Statements regarding our path to profitability constitute forward-looking statements, and are subject to numerous risks and assumptions. See slide 2 for more information. You should not rely upon forward-looking statements as predictions of future events
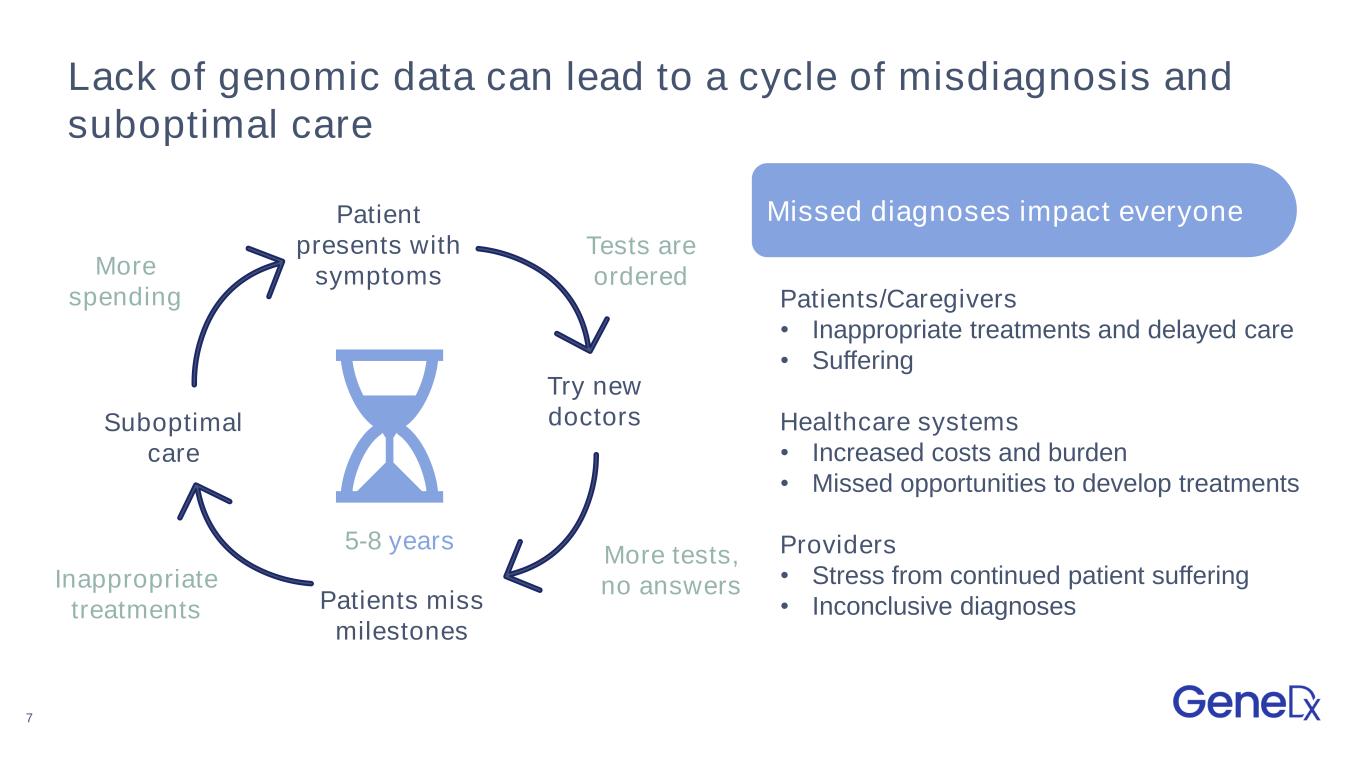
7 Lack of genomic data can lead to a cycle of misdiagnosis and suboptimal care Missed diagnoses impact everyone 5-8 years Patients/Caregivers • Inappropriate treatments and delayed care • Suffering Healthcare systems • Increased costs and burden • Missed opportunities to develop treatments Providers • Stress from continued patient suffering • Inconclusive diagnoses Try new doctors Patients miss milestones Patient presents with symptoms Suboptimal care More tests, no answersInappropriate treatments Tests are orderedMore spending

8 Genomic information is the most foundational health data source Each person's unique genetic code influences their health – and fundamentally informs how we use other data tools.

9 As a leader in exome and genome testing, GeneDx leverages the power of this data to: o Inform personalized health plans o accelerate drug discovery o improve efficiencies for health systems o enable healthier populations Exome and genome testing unlock the most comprehensive genomic data

GeneDx is positioned to enable a data-informed future for healthcare.
11 Leading exome and genome products o For more than 20 years, our advanced genomic technologies and unparalleled data have helped diagnose the hardest cases. o Now we’re helping patients with more common conditions harness the power of their genome and live healthier lives. Translating complex genomic data into definitive diagnoses for patients
12 Butler L. et al. Exome-based testing for patients with seizures: Advantages over panel-based testing. Poster presented at American Epilepsy Society Annual Meeting; December 2, 2023; Orlando, FL. Only 43% are tested on many commercial epilepsy panels Common diseases are in fact a constellation of genetic diagnoses One example is epilepsy. At least 768 different genes are related to seizures.
13 Butler L. et al. Exome-based testing for patients with seizures: Advantages over panel-based testing. Poster presented at American Epilepsy Society Annual Meeting; December 2, 2023; Orlando, FL. Exome sequencing checks all 768 genes Only 43% are tested on many commercial epilepsy panels Common diseases are in fact a constellation of genetic diagnoses One example is epilepsy. At least 768 different genes are related to seizures.
14 500K sequenced exomes Data is at the center of our business Our huge dataset and intelligent interpretation platform set us apart and fuel innovation Simplifies complex genomic data Reduces variants of unknown significance Increases diagnostic yield Significant clinical and genomic data Fuels improved testing accuracy Advances science and powering future discoveries Advanced interpretation platform
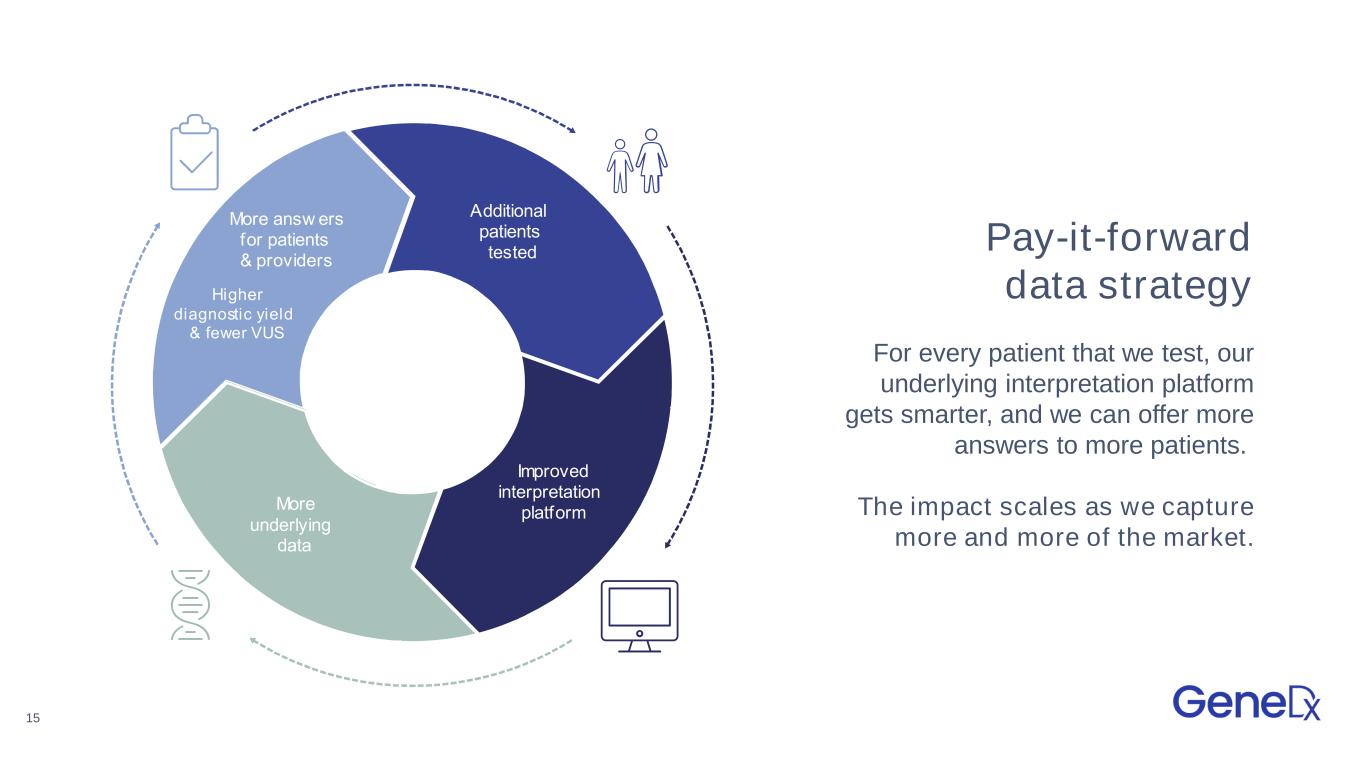
15 Pay-it-forward data strategy For every patient that we test, our underlying interpretation platform gets smarter, and we can offer more answers to more patients. The impact scales as we capture more and more of the market. dditional patients tested mproved interpretation platform More underlying data More answ ers for patients providers igher diagnostic y ield f ewer S dditional patients tested mproved interpretation platform More underlying data More answ ers for patients providers igher diagnostic yield fewer S
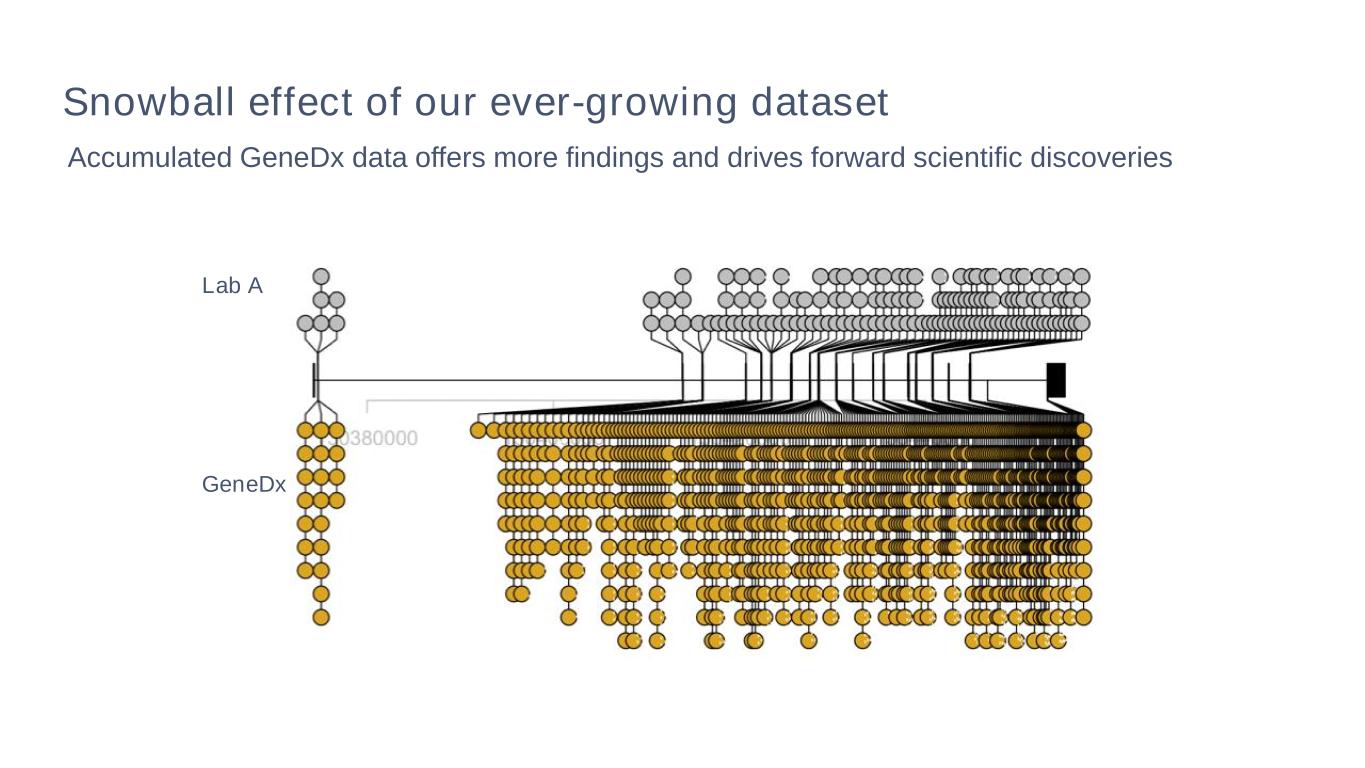
Accumulated GeneDx data offers more findings and drives forward scientific discoveries Lab A GeneDx Snowball effect of our ever-growing dataset

Exome and genome can be the best tests for patients. They're also the best tests for our business.

Today, we shorten the diagnostic journey. Tomorrow, we hope to prevent it.

19 Building the future: NICU Shorter hospital stays. Less uncertainty. Better care. 63% of infants had abnormal rapid WGS results, and 88% of these cases resulted in a change in management In phase one of the SeqFirst study, 125 infants were offered rapid WGS: 1 in 4 infants with abnormal results were not previously suspected to have a genetic syndrome Families of enrolled infants reported an overall positive experience, regardless of rapid WGS test outcome
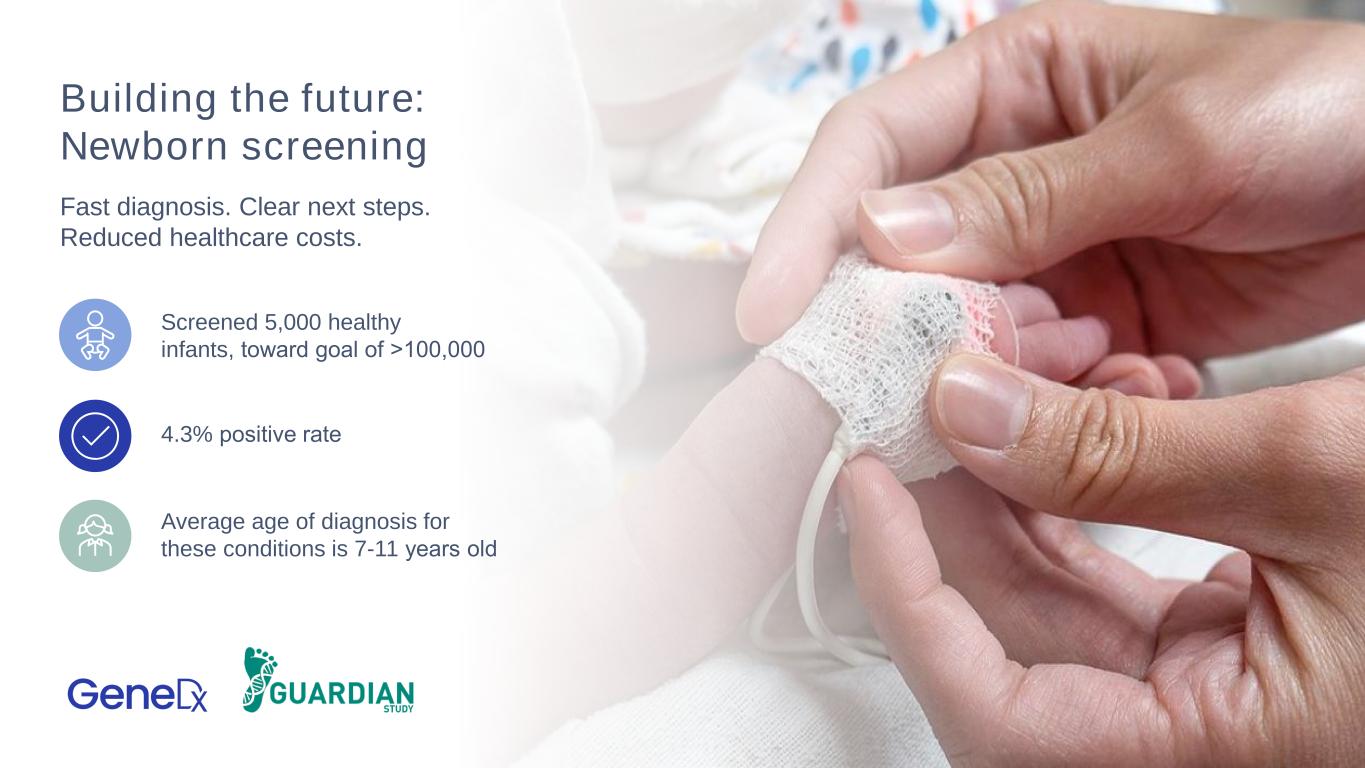
20 Building the future: Newborn screening Fast diagnosis. Clear next steps. Reduced healthcare costs. Screened 5,000 healthy infants, toward goal of >100,000 4.3% positive rate Average age of diagnosis for these conditions is 7-11 years old

21 Building the future: Partnerships Enriched data. Empowered drug discovery. mproved outcomes. GeneDx offers solutions across the pharma drug development pipeline Find Connect Understand
22 Building the future: Interpretation as a service Scientific rigor. Medical value. Establishing genomics as the standard of care for all.

23 All paths lead to The right organization. In the right space. At the right time.
v3.23.4
Document and Entity Information Document
|
Jan. 08, 2024 |
| Entity Information [Line Items] |
|
| Document Type |
8-K
|
| Document Period End Date |
Jan. 08, 2024
|
| Entity Registrant Name |
GeneDx Holdings Corp.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-39482
|
| Entity Tax Identification Number |
85-1966622
|
| Entity Address, Address Line One |
333 Ludlow Street
|
| Entity Address, City or Town |
Stamford
|
| Entity Address, State or Province |
CT
|
| Entity Address, Postal Zip Code |
06902
|
| City Area Code |
800
|
| Local Phone Number |
298-6470
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
true
|
| Entity Ex Transition Period |
false
|
| Entity Address, Address Line Two |
North Tower
|
| Entity Address, Address Line Three |
6th Floor
|
| Entity Central Index Key |
0001818331
|
| Amendment Flag |
false
|
| Common Class A |
|
| Entity Information [Line Items] |
|
| Title of 12(b) Security |
Class A common stock, par value $0.0001 per share
|
| Trading Symbol |
WGS
|
| Security Exchange Name |
NASDAQ
|
| Warrant |
|
| Entity Information [Line Items] |
|
| Title of 12(b) Security |
Warrants to purchase one share of Class A common stock, each at an exercise price of $379.50 per share
|
| Trading Symbol |
WGSWW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 3 such as an Office Park
| Name: |
dei_EntityAddressAddressLine3 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Grafico Azioni GeneDx (NASDAQ:WGS)
Storico
Da Nov 2024 a Dic 2024

Grafico Azioni GeneDx (NASDAQ:WGS)
Storico
Da Dic 2023 a Dic 2024
